Specific effects of potassium ion binding on wild-type and L358P cytochrome P450cam
- PMID: 17128977
- PMCID: PMC1764623
- DOI: 10.1021/bi0617355
Specific effects of potassium ion binding on wild-type and L358P cytochrome P450cam
Abstract
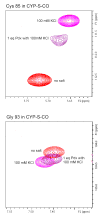
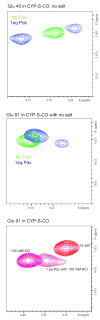
The camphor monoxygenase cytochrome P450cam (CYP101) requires potassium ion (K+) to drive formation of the characteristic high-spin state of the heme Fe+3 upon substrate binding. Amide 1H, 15N correlations in perdeuterated [U-15N] CYP101 were monitored as a function of K+ concentration by 2D-TROSY-HSQC in both camphor-bound oxidized (CYP-S) and camphor- and CO-bound reduced CYP101 (CYP-S-CO). In both forms, K+-induced spectral perturbations are detected in the vicinity of the K+ binding site proposed from crystallographic structures, but are larger and more widespread structurally in CYP-S than in CYP-S-CO. In CYP-S-CO, K+-induced perturbations occur primarily near the proposed K+ binding site in the B-B' loop and B' helix, which are also perturbed by binding of effector, putidaredoxin (Pdx). The spectral effects of K+ binding in CYP-S-CO oppose those observed upon Pdxr titration. However, Pdxr titration of CYP-S-CO in the absence of K+ results in multiple conformations. The spin-state equilibrium in the L358P mutant of CYP101 is more sensitive to K+ concentration than WT CYP101, consistent with a hypothesis that L358P preferentially populates conformations enforced by Pdx binding in WT CYP101. Thallium(I), a K+ mimic, minimizes the effects of Pdx titration on the NMR spectrum of CYP-S-CO, but is competent to replace K+ in driving the formation of high-spin CYP-S. These observations suggest that the role of K+ is to stabilize conformers of CYP-S that drive the spin-state change prior to the first electron transfer, and that K+ stabilizes the CYP-S-CO conformer that interacts with Pdx. However, upon binding of Pdx, further conformational changes occur that disfavor K+ binding.
Figures



Similar articles
-
Comparison of the complexes formed by cytochrome P450cam with cytochrome b5 and putidaredoxin, two effectors of camphor hydroxylase activity.Biochemistry. 2006 Mar 28;45(12):3887-97. doi: 10.1021/bi052318f. Biochemistry. 2006. PMID: 16548516 Free PMC article.
-
Detection of a high-barrier conformational change in the active site of cytochrome P450cam upon binding of putidaredoxin.J Am Chem Soc. 2005 May 18;127(19):6974-6. doi: 10.1021/ja051195j. J Am Chem Soc. 2005. PMID: 15884940 Free PMC article.
-
A model for effector activity in a highly specific biological electron transfer complex: the cytochrome P450(cam)-putidaredoxin couple.Biochemistry. 2003 May 20;42(19):5649-56. doi: 10.1021/bi034263s. Biochemistry. 2003. PMID: 12741821
-
Structural biology of redox partner interactions in P450cam monooxygenase: a fresh look at an old system.Arch Biochem Biophys. 2011 Mar 1;507(1):66-74. doi: 10.1016/j.abb.2010.08.022. Epub 2010 Sep 15. Arch Biochem Biophys. 2011. PMID: 20816746 Free PMC article. Review.
-
FTIR studies of the redox partner interaction in cytochrome P450: the Pdx-P450cam couple.Biochim Biophys Acta. 2007 Mar;1770(3):420-31. doi: 10.1016/j.bbagen.2006.08.020. Epub 2006 Sep 1. Biochim Biophys Acta. 2007. PMID: 17014964 Review.
Cited by
-
Experimentally restrained molecular dynamics simulations for characterizing the open states of cytochrome P450cam.Biochemistry. 2011 Mar 15;50(10):1664-71. doi: 10.1021/bi101820d. Epub 2011 Feb 8. Biochemistry. 2011. PMID: 21265500 Free PMC article.
-
Artificial self-sufficient P450 in reversed micelles.Molecules. 2010 Apr 27;15(5):2935-48. doi: 10.3390/molecules15052935. Molecules. 2010. PMID: 20657456 Free PMC article.
-
Dynamics underlying hydroxylation selectivity of cytochrome P450cam.Biophys J. 2021 Mar 2;120(5):912-923. doi: 10.1016/j.bpj.2021.01.027. Epub 2021 Feb 3. Biophys J. 2021. PMID: 33545101 Free PMC article.
-
Active Site Hydrogen Bonding Induced in Cytochrome P450cam by Effector Putidaredoxin.Biochemistry. 2021 Jun 1;60(21):1699-1707. doi: 10.1021/acs.biochem.1c00075. Epub 2021 May 18. Biochemistry. 2021. PMID: 34006086 Free PMC article.
-
A novel type of allosteric regulation: functional cooperativity in monomeric proteins.Arch Biochem Biophys. 2012 Mar 15;519(2):91-102. doi: 10.1016/j.abb.2011.12.017. Epub 2012 Jan 8. Arch Biochem Biophys. 2012. PMID: 22245335 Free PMC article. Review.
References
-
- Britt BM. For enzymes, bigger is better. Biophys Chem. 1997;69:63–70. - PubMed
-
- Mueller EJ, Loida PJ, Sligar SG. Cytochrome P450: Structure, Function and Biochemistry. 1995. pp. 83–124.
-
- Lange R, Bonfils C, Debey P. The low-spin reversible highspin transition equilibrium of camphor-bound cytochrome P-450. Effects of medium and temperature on equilibrium data. Eur J Biochem. 1977;79:623–628. - PubMed
-
- Lange R, Pierre J, Debey P. Visible and ultraviolet spectral transitions of camphor-bound cytochrome P-450. A comprehensive study. Eur J Biochem. 1980;107:441–445. - PubMed
-
- Hui Bon Hoa G, Marden MC. The pressure dependence of the spin equilibrium of camphor-bound cytochrome P450. Eur J Biochem. 1982;124:311–315. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

