The N-terminal domain of Bcl-xL reversibly binds membranes in a pH-dependent manner
- PMID: 17128992
- PMCID: PMC1764622
- DOI: 10.1021/bi0616652
The N-terminal domain of Bcl-xL reversibly binds membranes in a pH-dependent manner
Abstract
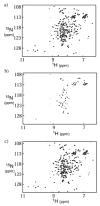
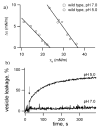
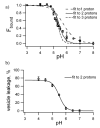
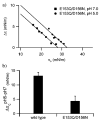
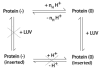
Bcl-xL regulates apoptosis by maintaining the integrity of the mitochondrial outer membrane by adopting both soluble and membrane-associated forms. The membrane-associated conformation does not require a conserved, C-terminal transmembrane domain and appears to be inserted into the bilayer of synthetic membranes as assessed by membrane permeabilization and critical surface pressure measurements. Membrane association is reversible and is regulated by the cooperative binding of approximately two protons to the protein. Two acidic residues, Glu153 and Asp156, that lie in a conserved hairpin of Bcl-xLDeltaTM appear to be important in this process on the basis of a 16% increase in the level of membrane association of the double mutant E153Q/D156N. Contrary to that for the wild type, membrane permeabilization for the mutant is not correlated with membrane association. Monolayer surface pressure measurements suggest that this effect is primarily due to less membrane penetration. These results suggest that E153 and D156 are important for the Bcl-xLDeltaTM conformational change and that membrane binding can be distinct from membrane permeabilization. Taken together, these studies support a model in which Bcl-xL activity is controlled by reversible insertion of its N-terminal domain into the mitochondrial outer membrane. Future studies with Bcl-xL mutants such as E153Q/D156N should allow determination of the relative contributions of membrane binding, insertion, and permeabilization to the regulation of apoptosis.
Figures



References
-
- Chao DT, Korsmeyer SJ. BCL-2 Family: Regulators of Cell Death. Annu Rev Immunol. 1998;16:395–419. - PubMed
-
- Gross A. BCL-2 Proteins: Regulators of the Mitochondrial Apoptotic Program. IUBMB Life. 2001;52:231–6. - PubMed
-
- Harris MH, Thompson CB. The Role of the Bcl-2 Family in the Regulation of Outer Mitochondrial Membrane Permeability. Cell Death Differ. 2000;7:1182–91. - PubMed
-
- Kuwana T, Newmeyer DD. Bcl-2-Family Proteins and the Role of Mitochondria in Apoptosis. Curr Opin Cell Biol. 2003;15:691–9. - PubMed
-
- Schendel SL, Montal M, Reed JC. Bcl-2 Family Proteins as Ion Channels. Cell Death Differ. 1998;5:372–80. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

