Lysosomes and Fas-mediated liver cell death
- PMID: 17129211
- PMCID: PMC1828900
- DOI: 10.1042/BJ20061738
Lysosomes and Fas-mediated liver cell death
Abstract
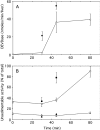
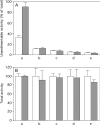
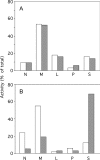
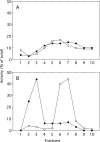
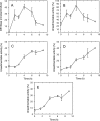
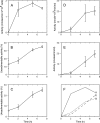
A number of studies, mostly performed ex vivo, suggest that lysosomes are involved in apoptosis as a result of a release of their cathepsins into the cytosol. These enzymes could then contribute to the permeabilization of the outer mitochondrial membrane; they could also activate effector caspases. The present study aims at testing whether the membrane of liver lysosomes is disrupted during Fas-mediated cell death of hepatocytes in vivo, a process implicated in several liver pathologies. Apoptosis was induced by injecting mice with aFas (anti-Fas antibody). The state of lysosomes was assessed by determining the proportion of lysosomal enzymes (beta-galactosidase, beta-glucuronidase, cathepsin C and cathepsin B) present in homogenate supernatants, devoid of intact lysosomes, and by analysing the behaviour in differential and isopycnic centrifugation of beta-galactosidase. Apoptosis was monitored by measuring caspase 3 activity (DEVDase) and the release of sulfite cytochrome c reductase, an enzyme located in the mitochondrial intermembrane space. Results show that an injection of 10 microg of aFas causes a rapid and large increase in DEVDase activity and in unsedimentable sulfite cytochrome c reductase. This modifies neither the proportion of unsedimentable lysosomal enzyme in the homogenates nor the behaviour of lysosomes in centrifugation. Experiments performed with a lower dose of aFas (5 microg) indicate that unsedimentable lysosomal hydrolase activity increases in the homogenate after injection but with a marked delay with respect to the increase in DEVDase activity and in unsedimentable sulfite cytochrome c reductase. Comparative experiments ex vivo performed with Jurkat cells show an increase in unsedimentable lysosomal hydrolases, but much later than caspase 3 activation, and a release of dipeptidyl peptidase III and DEVDase into culture medium. It is proposed that the weakening of lysosomes observed after aFas treatment in vivo and ex vivo results from a necrotic process that takes place late after initiation of apoptosis.
Figures
References
-
- Guicciardi M. E., Leist M., Gores G. J. Lysosomes in cell death. Oncogene. 2004;23:2881–2890. - PubMed
-
- Cirma T., Oresi K., Mazovec G. D., Turk V., Reed J. C., Myers R. M., Salvesen G. S., Turk B. Selective disruption of lysosomes in Hela cells triggers apoptosis mediated by cleavage of Bid by multiple papain-like lysosomal cathepsins. J. Biol. Chem. 2003;279:3578–3587. - PubMed
-
- Ferri K. F., Kroemer G. Organelle-specific initiation of cell death pathways. Nat. Cell Biol. 2001;3:E255–E263. - PubMed
-
- Kroemer G., Martin S. J. Caspase-independent cell-death. Nat. Med. 2005;11:725–730. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

