Novel self-epitopes derived from aggrecan, fibrillin, and matrix metalloproteinase-3 drive distinct autoreactive T-cell responses in juvenile idiopathic arthritis and in health
- PMID: 17129378
- PMCID: PMC1794523
- DOI: 10.1186/ar2088
Novel self-epitopes derived from aggrecan, fibrillin, and matrix metalloproteinase-3 drive distinct autoreactive T-cell responses in juvenile idiopathic arthritis and in health
Abstract
Juvenile idiopathic arthritis (JIA) is a heterogeneous autoimmune disease characterized by chronic joint inflammation. Knowing which antigens drive the autoreactive T-cell response in JIA is crucial for the understanding of disease pathogenesis and additionally may provide targets for antigen-specific immune therapy. In this study, we tested 9 self-peptides derived from joint-related autoantigens for T-cell recognition (T-cell proliferative responses and cytokine production) in 36 JIA patients and 15 healthy controls. Positive T-cell proliferative responses (stimulation index > or =2) to one or more peptides were detected in peripheral blood mononuclear cells (PBMC) of 69% of JIA patients irrespective of major histocompatibility complex (MHC) genotype. The peptides derived from aggrecan, fibrillin, and matrix metalloproteinase (MMP)-3 yielded the highest frequency of T-cell proliferative responses in JIA patients. In both the oligoarticular and polyarticular subtypes of JIA, the aggrecan peptide induced T-cell proliferative responses that were inversely related with disease duration. The fibrillin peptide, to our knowledge, is the first identified autoantigen that is primarily recognized in polyarticular JIA patients. Finally, the epitope derived from MMP-3 elicited immune responses in both subtypes of JIA and in healthy controls. Cytokine production in short-term peptide-specific T-cell lines revealed production of interferon-gamma (aggrecan/MMP-3) and interleukin (IL)-17 (aggrecan) and inhibition of IL-10 production (aggrecan). Here, we have identified a triplet of self-epitopes, each with distinct patterns of T-cell recognition in JIA patients. Additional experiments need to be performed to explore their qualities and role in disease pathogenesis in further detail.
Figures
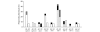
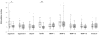



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

