Molecular subtypes of breast cancer in relation to paclitaxel response and outcomes in women with metastatic disease: results from CALGB 9342
- PMID: 17129383
- PMCID: PMC1797029
- DOI: 10.1186/bcr1622
Molecular subtypes of breast cancer in relation to paclitaxel response and outcomes in women with metastatic disease: results from CALGB 9342
Abstract
Introduction: The response to paclitaxel varies widely in metastatic breast cancer. We analyzed data from CALGB 9342, which tested three doses of paclitaxel in women with advanced disease, to determine whether response and outcomes differed according to HER2, hormone receptor, and p53 status.
Methods: Among 474 women randomly assigned to paclitaxel at a dose of 175, 210, or 250 mg/m2, adequate primary tumor tissue was available from 175. Immunohistochemistry with two antibodies and fluorescence in situ hybridization were performed to evaluate HER2 status; p53 status was determined by immunohistochemistry and sequencing. Hormone receptor status was obtained from pathology reports.
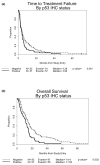
Results: Objective response rate was not associated with HER2 or p53 status. There was a trend toward a shorter median time to treatment failure among women with HER2-positive tumors (2.3 versus 4.2 months; P = 0.067). HER2 status was not related to overall survival (OS). Hormone receptor expression was not associated with differences in response but was associated with longer OS (P = 0.003). In contrast, women with p53 over-expression had significantly shorter OS than those without p53 over-expression (11.5 versus 14.4 months; P = 0.002). In addition, triple negative tumors were more frequent in African-American than in Caucasian patients, and were associated with a significant reduction in OS (8.7 versus 12.9 months; P = 0.008).
Conclusion: None of the biomarkers was predictive of treatment response in women with metastatic breast cancer; however, survival differed according to hormone receptor and p53 status. Triple negative tumors were more frequent in African-American patients and were associated with a shorter survival.
Figures

References
-
- Henderson IC, Berry DA, Demetri GD, Cirrincione CT, Goldstein LJ, Martino S, Ingle JN, Cooper MR, Hayes DF, Tkaczuk KH, et al. Improved outcomes from adding sequential paclitaxel but not from escalating doxorubicin dose in an adjuvant chemotherapy regimen for patients with node-positive primary breast cancer. J Clin Oncol. 2003;21:976–983. doi: 10.1200/JCO.2003.02.063. - DOI - PubMed
-
- Mamounas EP, Bryant J, Lembersky B, Fehrenbacher L, Sedlacek SM, Fisher B, Wickerham DL, Yothers G, Soran A, Wolmark N. Paclitaxel after doxorubicin plus cyclophosphamide as adjuvant chemotherapy for node-positive breast cancer: results from NSABP B-28. J Clin Oncol. 2005;23:3686–3696. doi: 10.1200/JCO.2005.10.517. - DOI - PubMed
-
- Reichman BS, Seidman AD, Crown JP, Heelan R, Hakes TB, Lebwohl DE, Gilewski TA, Surbone A, Currie V, Hudis CA, et al. Paclitaxel and recombinant human granulocyte colony-stimulating factor as initial chemotherapy for metastatic breast cancer. J Clin Oncol. 1993;11:1943–1951. - PubMed
-
- Seidman AD, Tiersten A, Hudis C, Gollub M, Barrett S, Yao TJ, Lepore J, Gilewski T, Currie V, Crown J, et al. Phase II trial of paclitaxel by 3-hour infusion as initial and salvage chemotherapy for metastatic breast cancer. J Clin Oncol. 1995;13:2575–2581. - PubMed
-
- Nabholtz JM, Gelmon K, Bontenbal M, Spielmann M, Catimel G, Conte P, Klaassen U, Namer M, Bonneterre J, Fumoleau P, Winograd B. Multicenter, randomized comparative study of two doses of paclitaxel in patients with metastatic breast cancer. J Clin Oncol. 1996;14:1858–1867. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

