Force sensing by mechanical extension of the Src family kinase substrate p130Cas
- PMID: 17129785
- PMCID: PMC2746973
- DOI: 10.1016/j.cell.2006.09.044
Force sensing by mechanical extension of the Src family kinase substrate p130Cas
Abstract
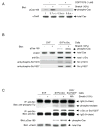
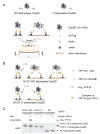
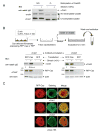
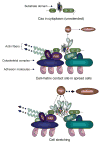
How physical force is sensed by cells and transduced into cellular signaling pathways is poorly understood. Previously, we showed that tyrosine phosphorylation of p130Cas (Cas) in a cytoskeletal complex is involved in force-dependent activation of the small GTPase Rap1. Here, we mechanically extended bacterially expressed Cas substrate domain protein (CasSD) in vitro and found a remarkable enhancement of phosphorylation by Src family kinases with no apparent change in kinase activity. Using an antibody that recognized extended CasSD in vitro, we observed Cas extension in intact cells in the peripheral regions of spreading cells, where higher traction forces are expected and where phosphorylated Cas was detected, suggesting that the in vitro extension and phosphorylation of CasSD are relevant to physiological force transduction. Thus, we propose that Cas acts as a primary force sensor, transducing force into mechanical extension and thereby priming phosphorylation and activation of downstream signaling.
Figures


Comment in
-
A role for p130Cas in mechanotransduction.Cell. 2006 Dec 1;127(5):879-81. doi: 10.1016/j.cell.2006.11.020. Cell. 2006. PMID: 17129774
References
-
- Balaban NQ, Schwarz US, Riveline D, Goichberg P, Tzur G, Sabanay I, Mahalu D, Safran S, Bershadsky A, Addadi L, Geiger B. Force and focal adhesion assembly: a close relationship studied using elastic micropatterned substrates. Nat Cell Biol. 2001;3:466–472. - PubMed
-
- Ballestrem C, Erez N, Kirchner J, Kam Z, Bershadsky A, Geiger B. Molecular mapping of tyrosine-phosphorylated proteins in focal adhesions using fluorescence resonance energy transfer. J Cell Sci. 2006;119:866–875. - PubMed
-
- Bougeret C, Rothhut B, Jullien P, Fischer S, Benarous R. Recombinant Csk expressed in Escherichia coli is autophosphorylated on tyrosine residue(s) Oncogene. 1993;8:1241–1247. - PubMed
-
- Briknarova K, Nasertorabi F, Havert ML, Eggleston E, Hoyt DW, Li C, Olson AJ, Vuori K, Ely KR. The serine-rich domain from Crk-associated substrate (p130Cas) is a four-helix bundle. J Biol Chem. 2005;280:21908–21914. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

