The natural history of the WRKY-GCM1 zinc fingers and the relationship between transcription factors and transposons
- PMID: 17130173
- PMCID: PMC1702500
- DOI: 10.1093/nar/gkl888
The natural history of the WRKY-GCM1 zinc fingers and the relationship between transcription factors and transposons
Abstract
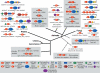
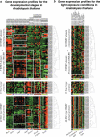
WRKY and GCM1 are metal chelating DNA-binding domains (DBD) which share a four stranded fold. Using sensitive sequence searches, we show that this WRKY-GCM1 fold is also shared by the FLYWCH Zn-finger domain and the DBDs of two classes of Mutator-like element (MULE) transposases. We present evidence that they share a stabilizing core, which suggests a possible origin from a BED finger-like intermediate that was in turn ultimately derived from a C2H2 Zn-finger domain. Through a systematic study of the phyletic pattern, we show that this WRKY-GCM1 superfamily is a widespread eukaryote-specific group of transcription factors (TFs). We identified several new members across diverse eukaryotic lineages, including potential TFs in animals, fungi and Entamoeba. By integrating sequence, structure, gene expression and transcriptional network data, we present evidence that at least two major global regulators belonging to this superfamily in Saccharomyces cerevisiae (Rcs1p and Aft2p) have evolved from transposons, and attained the status of transcription regulatory hubs in recent course of ascomycete yeast evolution. In plants, we show that the lineage-specific expansion of WRKY-GCM1 domain proteins acquired functional diversity mainly through expression divergence rather than by protein sequence divergence. We also use the WRKY-GCM1 superfamily as an example to illustrate the importance of transposons in the emergence of new TFs in different lineages.
Figures



Similar articles
-
Phantom, a new subclass of Mutator DNA transposons found in insect viruses and widely distributed in animals.Genetics. 2010 Aug;185(4):1507-17. doi: 10.1534/genetics.110.116673. Epub 2010 May 10. Genetics. 2010. PMID: 20457878 Free PMC article.
-
Mutator-like element in the yeast Yarrowia lipolytica displays multiple alternative splicings.Eukaryot Cell. 2005 Mar;4(3):615-24. doi: 10.1128/EC.4.3.615-624.2005. Eukaryot Cell. 2005. PMID: 15755923 Free PMC article.
-
Curupira-1 and Curupira-2, two novel Mutator-like DNA transposons from the genomes of human parasites Schistosoma mansoni and Schistosoma japonicum.Parasitology. 2011 Aug;138(9):1124-33. doi: 10.1017/S0031182011000886. Epub 2011 Jul 15. Parasitology. 2011. PMID: 21756422
-
DNA-binding domains of plant-specific transcription factors: structure, function, and evolution.Trends Plant Sci. 2013 May;18(5):267-76. doi: 10.1016/j.tplants.2012.09.001. Epub 2012 Oct 3. Trends Plant Sci. 2013. PMID: 23040085 Review.
-
[Structure and function of plant C2H2 zinc finger protein].Yi Chuan. 2004 May;26(3):414-8. Yi Chuan. 2004. PMID: 15640031 Review. Chinese.
Cited by
-
Monothiol CGFS glutaredoxins and BolA-like proteins: [2Fe-2S] binding partners in iron homeostasis.Biochemistry. 2012 Jun 5;51(22):4377-89. doi: 10.1021/bi300393z. Epub 2012 May 23. Biochemistry. 2012. PMID: 22583368 Free PMC article. Review.
-
A widespread occurrence of extra open reading frames in plant Ty3/gypsy retrotransposons.Genetica. 2011 Dec;139(11-12):1543-55. doi: 10.1007/s10709-012-9654-9. Epub 2012 Apr 29. Genetica. 2011. PMID: 22544262
-
The Sir2-Sum1 complex represses transcription using both promoter-specific and long-range mechanisms to regulate cell identity and sexual cycle in the yeast Kluyveromyces lactis.PLoS Genet. 2009 Nov;5(11):e1000710. doi: 10.1371/journal.pgen.1000710. Epub 2009 Nov 6. PLoS Genet. 2009. PMID: 19893609 Free PMC article.
-
Transcription factors involved in basal immunity in mammals and plants interact with the same MAMP-responsive cis-sequence from Arabidopsis thaliana.Plant Mol Biol. 2018 Dec;98(6):565-578. doi: 10.1007/s11103-018-0796-8. Epub 2018 Nov 22. Plant Mol Biol. 2018. PMID: 30467788
-
Functional characterization of the active Mutator-like transposable element, Muta1 from the mosquito Aedes aegypti.Mob DNA. 2017 Jan 11;8:1. doi: 10.1186/s13100-016-0084-6. eCollection 2017. Mob DNA. 2017. PMID: 28096902 Free PMC article.
References
-
- Lindahl L., Hinnebusch A. Diversity of mechanisms in the regulation of translation in prokaryotes and lower eukaryotes. Curr. Opin. Genet. Dev. 1992;2:720–726. - PubMed
-
- Aravind L., Anantharaman V., Balaji S., Babu M.M., Iyer L.M. The many faces of the helix–turn–helix domain: transcription regulation and beyond. FEMS Microbiol Rev. 2005;29:231–262. - PubMed
-
- Babu M.M., Luscombe N.M., Aravind L., Gerstein M., Teichmann S.A. Structure and evolution of transcriptional regulatory networks. Curr. Opin. Struct. Biol. 2004;14:283–291. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

