Unassisted translocation of large polypeptide domains across phospholipid bilayers
- PMID: 17130291
- PMCID: PMC2064676
- DOI: 10.1083/jcb.200608101
Unassisted translocation of large polypeptide domains across phospholipid bilayers
Abstract
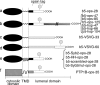
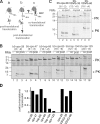
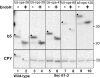
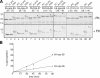
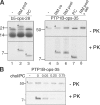
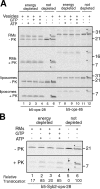
Although transmembrane proteins generally require membrane-embedded machinery for integration, a few can insert spontaneously into liposomes. Previously, we established that the tail-anchored (TA) protein cytochrome b(5) (b5) can posttranslationally translocate 28 residues downstream to its transmembrane domain (TMD) across protein-free bilayers (Brambillasca, S., M. Yabal, P. Soffientini, S. Stefanovic, M. Makarow, R.S. Hegde, and N. Borgese. 2005. EMBO J. 24:2533-2542). In the present study, we investigated the limits of this unassisted translocation and report that surprisingly long (85 residues) domains of different sequence and charge placed downstream of b5's TMD can posttranslationally translocate into mammalian microsomes and liposomes at nanomolar nucleotide concentrations. Furthermore, integration of these constructs occurred in vivo in translocon-defective yeast strains. Unassisted translocation was not unique to b5 but was also observed for another TA protein (protein tyrosine phosphatase 1B) whose TMD, like the one of b5, is only moderately hydrophobic. In contrast, more hydrophobic TMDs, like synaptobrevin's, were incapable of supporting unassisted integration, possibly because of their tendency to aggregate in aqueous solution. Our data resolve long-standing discrepancies on TA protein insertion and are relevant to membrane evolution, biogenesis, and physiology.
Figures

Similar articles
-
Helix insertion into bilayers and the evolution of membrane proteins.Cell Mol Life Sci. 2010 Apr;67(7):1077-88. doi: 10.1007/s00018-009-0234-9. Epub 2009 Dec 29. Cell Mol Life Sci. 2010. PMID: 20039094 Free PMC article. Review.
-
The role of cytosolic proteins in the insertion of tail-anchored proteins into phospholipid bilayers.J Cell Sci. 2009 Jul 15;122(Pt 14):2383-92. doi: 10.1242/jcs.049460. Epub 2009 Jun 16. J Cell Sci. 2009. PMID: 19531581
-
Translocation of the C terminus of a tail-anchored protein across the endoplasmic reticulum membrane in yeast mutants defective in signal peptide-driven translocation.J Biol Chem. 2003 Jan 31;278(5):3489-96. doi: 10.1074/jbc.M210253200. Epub 2002 Nov 22. J Biol Chem. 2003. PMID: 12446686
-
ATR-FTIR study of the structure and orientation of transmembrane domains of the Saccharomyces cerevisiae alpha-mating factor receptor in phospholipids.Biochemistry. 2001 Jul 31;40(30):8945-54. doi: 10.1021/bi010394m. Biochemistry. 2001. PMID: 11467956
-
Protein structure in membrane domains.Annu Rev Biophys. 2012;41:135-55. doi: 10.1146/annurev-biophys-050511-102310. Annu Rev Biophys. 2012. PMID: 22577820 Review.
Cited by
-
Endoplasmic Reticulum Membrane Homeostasis and the Unfolded Protein Response.Cold Spring Harb Perspect Biol. 2024 Aug 1;16(8):a041400. doi: 10.1101/cshperspect.a041400. Cold Spring Harb Perspect Biol. 2024. PMID: 38253414 Free PMC article. Review.
-
Distinct pathways mediate the sorting of tail-anchored proteins to the plastid outer envelope.PLoS One. 2010 Apr 14;5(4):e10098. doi: 10.1371/journal.pone.0010098. PLoS One. 2010. PMID: 20418952 Free PMC article.
-
Helix insertion into bilayers and the evolution of membrane proteins.Cell Mol Life Sci. 2010 Apr;67(7):1077-88. doi: 10.1007/s00018-009-0234-9. Epub 2009 Dec 29. Cell Mol Life Sci. 2010. PMID: 20039094 Free PMC article. Review.
-
EMC rectifies the topology of multipass membrane proteins.Nat Struct Mol Biol. 2024 Jan;31(1):32-41. doi: 10.1038/s41594-023-01120-6. Epub 2023 Nov 13. Nat Struct Mol Biol. 2024. PMID: 37957425 Free PMC article.
-
Viroporins, Examples of the Two-Stage Membrane Protein Folding Model.Viruses. 2015 Jun 26;7(7):3462-82. doi: 10.3390/v7072781. Viruses. 2015. PMID: 26131957 Free PMC article. Review.
References
-
- Abell, B.M., M. Jung, J.D. Oliver, B.C. Knight, J. Tyedmers, R. Zimmermann, and S. High. 2003. Tail-anchored and signal-anchored proteins utilise overlapping pathways during membrane insertion. J. Biol. Chem. 278:5669–5678. - PubMed
-
- Ames, B.N., and D.T. Dubin. 1960. The role of polyamines in the neutralization of bacteriophage deoxyribonucleic acid. J. Biol. Chem. 235:769–775. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

