A cation-pi interaction between extracellular TEA and an aromatic residue in potassium channels
- PMID: 17130518
- PMCID: PMC2151593
- DOI: 10.1085/jgp.200609654
A cation-pi interaction between extracellular TEA and an aromatic residue in potassium channels
Abstract
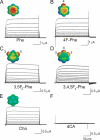
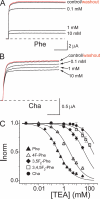
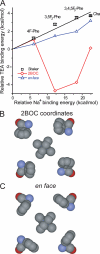
Open-channel blockers such as tetraethylammonium (TEA) have a long history as probes of the permeation pathway of ion channels. High affinity blockade by extracellular TEA requires the presence of an aromatic amino acid at a position that sits at the external entrance of the permeation pathway (residue 449 in the eukaryotic voltage-gated potassium channel Shaker). We investigated whether a cation-pi interaction between TEA and such an aromatic residue contributes to TEA block using the in vivo nonsense suppression method to incorporate a series of increasingly fluorinated Phe side chains at position 449. Fluorination, which is known to decrease the cation-pi binding ability of an aromatic ring, progressively increased the inhibitory constant K(i) for the TEA block of Shaker. A larger increase in K(i) was observed when the benzene ring of Phe449 was substituted by nonaromatic cyclohexane. These results support a strong cation-pi component to the TEA block. The data provide an empirical basis for choosing between Shaker models that are based on two classes of reported crystal structures for the bacterial channel KcsA, showing residue Tyr82 in orientations either compatible or incompatible with a cation-pi mechanism. We propose that the aromatic residue at this position in Shaker is favorably oriented for a cation-pi interaction with the permeation pathway. This choice is supported by high level ab initio calculations of the predicted effects of Phe modifications on TEA binding energy.
Figures
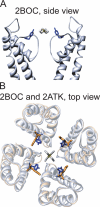

Comment in
-
Extracellular blockade of potassium channels by TEA+: the tip of the iceberg?J Gen Physiol. 2006 Dec;128(6):635-6. doi: 10.1085/jgp.200609684. J Gen Physiol. 2006. PMID: 17130517 Free PMC article. No abstract available.
References
-
- Beene, D.L., G.S. Brandt, W. Zhong, N.M. Zacharias, H.A. Lester, and D.A. Dougherty. 2002. Cation-pi interactions in ligand recognition by serotonergic (5-HT3A) and nicotinic acetylcholine receptors: the anomalous binding properties of nicotine. Biochemistry. 41:10262–10269. - PubMed
-
- Brejc, K., W. J. van Dijk, R.V. Klaassen, M. Schuurmans, J. van Der Oost, A.B. Smit, and T.K. Sixma. 2001. Crystal structure of an ACh-binding protein reveals the ligand-binding domain of nicotinic receptors. Nature. 411:269–276. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

