An S6 mutation in BK channels reveals beta1 subunit effects on intrinsic and voltage-dependent gating
- PMID: 17130522
- PMCID: PMC2151602
- DOI: 10.1085/jgp.200609596
An S6 mutation in BK channels reveals beta1 subunit effects on intrinsic and voltage-dependent gating
Abstract
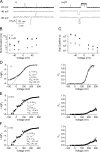
Large conductance, Ca(2+)- and voltage-activated K(+) (BK) channels are exquisitely regulated to suit their diverse roles in a large variety of physiological processes. BK channels are composed of pore-forming alpha subunits and a family of tissue-specific accessory beta subunits. The smooth muscle-specific beta1 subunit has an essential role in regulating smooth muscle contraction and modulates BK channel steady-state open probability and gating kinetics. Effects of beta1 on channel's gating energetics are not completely understood. One of the difficulties is that it has not yet been possible to measure the effects of beta1 on channel's intrinsic closed-to-open transition (in the absence of voltage sensor activation and Ca(2+) binding) due to the very low open probability in the presence of beta1. In this study, we used a mutation of the alpha subunit (F315Y) that increases channel openings by greater than four orders of magnitude to directly compare channels' intrinsic open probabilities in the presence and absence of the beta1 subunit. Effects of beta1 on steady-state open probabilities of both wild-type alpha and the F315Y mutation were analyzed using the dual allosteric HA model. We found that mouse beta1 has two major effects on channel's gating energetics. beta1 reduces the intrinsic closed-to-open equilibrium that underlies the inhibition of BK channel opening seen in submicromolar Ca(2+). Further, P(O) measurements at limiting slope allow us to infer that beta1 shifts open channel voltage sensor activation to negative membrane potentials, which contributes to enhanced channel opening seen at micromolar Ca(2+) concentrations. Using the F315Y alpha subunit with deletion mutants of beta1, we also demonstrate that the small N- and C-terminal intracellular domains of beta1 play important roles in altering channel's intrinsic opening and voltage sensor activation. In summary, these results demonstrate that beta1 has distinct effects on BK channel intrinsic gating and voltage sensor activation that can be functionally uncoupled by mutations in the intracellular domains.
Figures


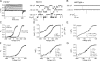




Similar articles
-
A role for the S0 transmembrane segment in voltage-dependent gating of BK channels.J Gen Physiol. 2007 Mar;129(3):209-20. doi: 10.1085/jgp.200609662. Epub 2007 Feb 12. J Gen Physiol. 2007. PMID: 17296928 Free PMC article.
-
Gating and ionic currents reveal how the BKCa channel's Ca2+ sensitivity is enhanced by its beta1 subunit.J Gen Physiol. 2005 Oct;126(4):393-412. doi: 10.1085/jgp.200509346. J Gen Physiol. 2005. PMID: 16186565 Free PMC article.
-
Mechanism of beta4 subunit modulation of BK channels.J Gen Physiol. 2006 Apr;127(4):449-65. doi: 10.1085/jgp.200509436. J Gen Physiol. 2006. PMID: 16567466 Free PMC article.
-
Large conductance Ca2+-activated K+ (BK) channel: activation by Ca2+ and voltage.Biol Res. 2006;39(3):385-401. doi: 10.4067/s0716-97602006000300003. Epub 2006 Nov 7. Biol Res. 2006. PMID: 17106573 Review.
-
Molecular mechanisms of BK channel activation.Cell Mol Life Sci. 2009 Mar;66(5):852-75. doi: 10.1007/s00018-008-8609-x. Cell Mol Life Sci. 2009. PMID: 19099186 Free PMC article. Review.
Cited by
-
Modulation of BK Channel Function by Auxiliary Beta and Gamma Subunits.Int Rev Neurobiol. 2016;128:51-90. doi: 10.1016/bs.irn.2016.03.015. Epub 2016 Apr 8. Int Rev Neurobiol. 2016. PMID: 27238261 Free PMC article. Review.
-
Modulation of the conductance-voltage relationship of the BK Ca channel by mutations at the putative flexible interface between two RCK domains.Biophys J. 2008 Jan 15;94(2):446-56. doi: 10.1529/biophysj.107.108738. Epub 2007 Sep 21. Biophys J. 2008. PMID: 17890381 Free PMC article.
-
A role for the S0 transmembrane segment in voltage-dependent gating of BK channels.J Gen Physiol. 2007 Mar;129(3):209-20. doi: 10.1085/jgp.200609662. Epub 2007 Feb 12. J Gen Physiol. 2007. PMID: 17296928 Free PMC article.
-
Modulation of BK channel voltage gating by different auxiliary β subunits.Proc Natl Acad Sci U S A. 2012 Nov 13;109(46):18991-6. doi: 10.1073/pnas.1216953109. Epub 2012 Oct 29. Proc Natl Acad Sci U S A. 2012. PMID: 23112204 Free PMC article.
-
An extracellular domain of the accessory β1 subunit is required for modulating BK channel voltage sensor and gate.J Gen Physiol. 2012 Jan;139(1):57-67. doi: 10.1085/jgp.201110698. Epub 2011 Dec 12. J Gen Physiol. 2012. PMID: 22155735 Free PMC article.
References
-
- Brenner, R., T.J. Jegla, A. Wickenden, Y. Liu, and R.W. Aldrich. 2000. a. Cloning and functional characterization of novel large conductance calcium-activated potassium channel β subunits, hKCNMB3 and hKCNMB4. J. Biol. Chem. 275:6453–6461. - PubMed
-
- Brenner, R., G.J. Perez, A.D. Bonev, D.M. Eckman, J.C. Kosek, S.W. Wiler, A.J. Patterson, M.T. Nelson, and R.W. Aldrich. 2000. b. Vasoregulation by the β1 subunit of the calcium-activated potassium channel. Nature. 407:870–876. - PubMed
-
- Brenner, R., Q.H. Chen, A. Vilaythong, G.M. Toney, J.L. Noebels, and R.W. Aldrich. 2005. BK channel β4 subunit reduces dentate gyrus excitability and protects against temporal lobe seizures. Nat. Neurosci. 8:1752–1759. - PubMed
-
- Butler, A., S. Tsunoda, D.P. McCobb, A. Wei, and L. Salkoff. 1993. mSlo, a complex mouse gene encoding “maxi” calcium-activated potassium channels. Science. 261:221–224. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

