In the FVB/N HER-2/neu transgenic mouse both peripheral and central tolerance limit the immune response targeting HER-2/neu induced by Listeria monocytogenes-based vaccines
- PMID: 17131121
- PMCID: PMC11030683
- DOI: 10.1007/s00262-006-0237-4
In the FVB/N HER-2/neu transgenic mouse both peripheral and central tolerance limit the immune response targeting HER-2/neu induced by Listeria monocytogenes-based vaccines
Abstract
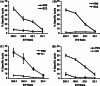
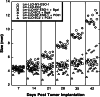
Listeria monocytogenes-based vaccines for HER-2/neu are capable of breaking tolerance in FVB/N rat HER-2/neu transgenic mice. The growth of implanted NT-2 tumors, derived from a spontaneously occurring tumor in the FVB/N HER-2/neu transgenic mouse, was significantly slower in these mice following vaccination with a series of L. monocytogenes-based vaccines for HER-2/neu. Mechanisms of T cell tolerance that exist in these transgenic mice include the absence of functional high avidity anti-HER-2/neu CD8(+) T cells and the presence of CD4(+)CD25(+) regulatory T cells. The in vivo depletion of these regulatory T cells resulted in the slowing in growth of tumors even without the treatment of mice with an anti-HER-2/neu vaccine. The average avidities of responsive CD8(+) T cells to six of the nine epitopes in HER-2/neu we examined, four of which were identified in this study, are shown here to be of a lower average avidity in the transgenic mice versus wild type FVB/N mice. In contrast, the average avidity of CD8(+) T cells to three epitopes that showed the lowest avidity in the wild-type mice did not differ between wild type and transgenic mice. This study demonstrates the ability of L. monocytogenes-based vaccines to impact upon tolerance to HER-2/neu in FVB/N HER-2/neu transgenic mice and further defines some of the aspects of tolerance in these mice.
Figures



References
-
- Chattopadhyay S, Mehrotra S, Chhabra A, Hegde U, Mukherji B, Chakraborty NG. Effect of CD4+CD25+ and CD4+CD25- T regulatory cells on the generation of cytolytic T cell response to a self but human tumor-associated epitope in vitro. J Immunol. 2006;176:984. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

