Effect of Kaiyu Qingwei Jianji on the morphometry and residual strain distribution of small intestine in experimental diabetic rats
- PMID: 17131477
- PMCID: PMC4087776
- DOI: 10.3748/wjg.v12.i44.7149
Effect of Kaiyu Qingwei Jianji on the morphometry and residual strain distribution of small intestine in experimental diabetic rats
Abstract
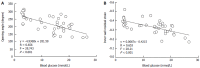
Aim: To investigate the effect of a Chinese medicine, Kaiyu Qingwei Jianji (KYQWJJ) used for diabetic treatment, on the morphometry and residual strain distribution of the small intestine in streptozotocin (STZ) -induced diabetic rats. Correlation analysis was also performed between the opening angle and residual strain with the blood glucose level.
Methods: Forty-two male Wistar rats weighing 220-240 g were included in this study. Thirty-two STZ-induced diabetic rats were subdivided into four groups (n = 8 in each group), i.e. diabetic control group (DM); high dose of KYQWJJ (T1, 36 g/kg per day); low dose of KYQWJJ (T2, 17 g/kg per day) and Gliclazide (T3, 50 mg/kg per day). Another ten rats were used as non-diabetic control (CON). The medicines were poured directly into stomach lumen by gastric lavage twice daily. The rats of CON and DM groups were only poured the physiological saline. Blood glucose and plasma insulin levels were measured. Experimental period was 35 d. At the end of experiment, three 5-cm long segments were harvested from the duodenum, jejunum and ileum. Three rings of 1-2 mm in length for no-load and zero-stress state tests were cut from the middle of different segments. The morphometric data, such as the circumferential length, the wall thickness and the opening angle were measured from the digitized images of intestinal segments in the no-load state and zero-stress state. The residual strain was computed from the morphometry data. Furthermore, the linear regression analysis was performed between blood glucose level with morphometric and biomechanical data in the different intestinal segments.
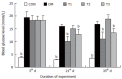
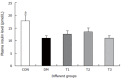
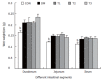
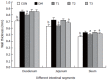
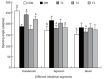
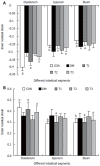
Results: The blood glucose level of DM group was consistent 4-fold to 5-fold higher than those in CON group during the experiment (16.89+/-1.11 vs 3.44+/-0.15 mmol/L, P < 0.001). The blood glucose level in the T1 (16.89+/-1.11 vs 11.08+/-2.67 mmol/L, P < 0.01) and T3 groups (16.89+/-1.11 vs 13.54+/-1.73 mmol/L, P < 0.05), but not in T2 group (P > 0.05) was significantly lower than those in DM group. The plasma insulin levels of DM, T1, T2 and T3 groups were significantly lower than those in CON group (10.98+/-1.02, 12.52+/-1.42,13.54+/-1.56,10.96+/-0.96 vs 17.84+/-2.34 pmol/L respectively, P < 0.05), but no significantly difference among the groups with exception of CON group. The wet weight/cm and total wall thickness of duodenum, jejunum and ileum in DM group were significantly higher than those in CON group (wet weight (g/cm): duodenum 0.209+/-0.012 vs 0.166+/-0.010, jejunum 0.149+/-0.008 vs 0.121+/-0.004, ileum 0.134+/-0.013 vs 0.112+/-0.007; Wall thickness (mm): duodenum 0.849+/-0.027 vs 0.710+/-0.026, jejunum 0.7259+/-0.034 vs 0.627+/-0.025, ileum 0.532+/-0.023 vs 0.470+/-0.010, all P < 0.05), T1 and T3 treatment could partly restore change of wall thickness, but T2 could not. The opening angle and absolute value of inner and outer residual stain were significantly smaller in duodenal segment (188+/-11 degrees, -0.31+/-0.02 and 0.35+/-0.03 vs 259+/-15 degrees, -0.40+/-0.02 and 0.43+/-0.05) and larger in jejunal (215+/-20 degrees, -0.30+/-0.03 and 0.36+/-0.06 vs 172+/-19 degrees, -0.25+/-0.02 and 0.27+/-0.02) and ileal segments (183+/-20 degrees, -0.28+/-0.01 and 0.34+/-0.05 vs 153+/-14 degrees, -0.23+/-0.03 and 0.29+/-0.04) in DM group than in CON group (P < 0.01). T1 and T3 treatment could partly restore this biomechanical alteration, but strong effect was found in T1 treatment (duodenum 243+/-14 degrees, -0.36+/-0.02 and 0.42+/-0.06, jejunum 180+/-15 degrees, -0.26+/-0.03 and 0.30+/-0.06 and ileum 163+/-17 degrees, -0.23+/-0.03 and 0.30+/-0.05, compared with DM, P < 0.05). The linear association was found between the glucose level with most morphometric and biomechanical data.
Conclusion: KYQWJJ (high dose) treatment could partly restore the changes of blood glucose level and the remodeling of morphometry and residual strain of small intestine in diabetic rats. The linear regression analysis demonstrated that the effect of KYQWJJ on intestinal opening angle and residual strain is partially through its effect on the blood glucose level.
Figures
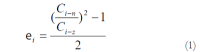
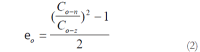
Similar articles
-
Effect of tangweian jianji on upper gastrointestinal remodeling in streptozotocin-induced diabetic rats.World J Gastroenterol. 2012 Sep 21;18(35):4875-84. doi: 10.3748/wjg.v18.i35.4875. World J Gastroenterol. 2012. PMID: 23002359 Free PMC article.
-
Remodelling of zero-stress state of small intestine in streptozotocin-induced diabetic rats. Effect of gliclazide.Dig Liver Dis. 2002 Oct;34(10):707-16. doi: 10.1016/s1590-8658(02)80022-6. Dig Liver Dis. 2002. PMID: 12469798
-
Biomechanical and morphometric intestinal remodelling during experimental diabetes in rats.Diabetologia. 2003 Dec;46(12):1688-97. doi: 10.1007/s00125-003-1233-2. Epub 2003 Oct 31. Diabetologia. 2003. PMID: 14593459
-
Photobiomodulation in diabetic rats: Effects on morphological, pancreatic parameters, and glucose homeostasis.J Biophotonics. 2023 Nov;16(11):e202300182. doi: 10.1002/jbio.202300182. Epub 2023 Aug 22. J Biophotonics. 2023. PMID: 37528614 Review.
-
What are the residual stresses doing in our blood vessels?Ann Biomed Eng. 1991;19(3):237-49. doi: 10.1007/BF02584301. Ann Biomed Eng. 1991. PMID: 1928868 Review.
Cited by
-
Gastric Emptying Time and Volume of the Small Intestine as Objective Markers in Patients With Symptoms of Diabetic Enteropathy.J Neurogastroenterol Motil. 2021 Jul 30;27(3):390-399. doi: 10.5056/jnm19195. J Neurogastroenterol Motil. 2021. PMID: 34210904 Free PMC article.
-
Characterization of the layer, direction and time-dependent mechanical behaviour of the human oesophagus and the effects of formalin preservation.J R Soc Interface. 2024 Apr;21(213):20230592. doi: 10.1098/rsif.2023.0592. Epub 2024 Apr 10. J R Soc Interface. 2024. PMID: 38593841 Free PMC article.
-
Diabetes-induced mechanophysiological changes in the small intestine and colon.World J Diabetes. 2017 Jun 15;8(6):249-269. doi: 10.4239/wjd.v8.i6.249. World J Diabetes. 2017. PMID: 28694926 Free PMC article. Review.
-
Supplementation with L-Glutamine and L-Alanyl-L-Glutamine Changes Biochemical Parameters and Jejunum Morphophysiology in Type 1 Diabetic Wistar Rats.PLoS One. 2015 Dec 14;10(12):e0143005. doi: 10.1371/journal.pone.0143005. eCollection 2015. PLoS One. 2015. PMID: 26659064 Free PMC article.
-
Effect of Tangshen formula on the remodeling of small intestine and colon in Zucker diabetic fatty rats.Heliyon. 2023 Oct 16;9(10):e21007. doi: 10.1016/j.heliyon.2023.e21007. eCollection 2023 Oct. Heliyon. 2023. PMID: 37886764 Free PMC article.
References
-
- Folwaczny C, Riepl R, Tschöp M, Landgraf R. Gastrointestinal involvement in patients with diabetes mellitus: Part I (first of two parts). Epidemiology, pathophysiology, clinical findings. Z Gastroenterol. 1999;37:803–815. - PubMed
-
- Verne GN, Sninsky CA. Diabetes and the gastrointestinal tract. Gastroenterol Clin North Am. 1998;27:861–74, vi-vii. - PubMed
-
- Zoubi SA, Williams MD, Mayhew TM, Sparrow RA. Number and ultrastructure of epithelial cells in crypts and villi along the streptozotocin-diabetic small intestine: a quantitative study on the effects of insulin and aldose reductase inhibition. Virchows Arch. 1995;427:187–193. - PubMed
-
- Mayhew TM, Carson FL, Sharma AK. Small intestinal morphology in experimental diabetic rats: a stereological study on the effects of an aldose reductase inhibitor (ponalrestat) given with or without conventional insulin therapy. Diabetologia. 1989;32:649–654. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

