Autophagy counterbalances endoplasmic reticulum expansion during the unfolded protein response
- PMID: 17132049
- PMCID: PMC1661684
- DOI: 10.1371/journal.pbio.0040423
Autophagy counterbalances endoplasmic reticulum expansion during the unfolded protein response
Abstract
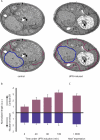
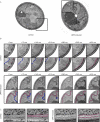
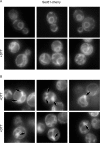
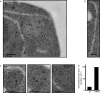
The protein folding capacity of the endoplasmic reticulum (ER) is regulated by the unfolded protein response (UPR). The UPR senses unfolded proteins in the ER lumen and transmits that information to the cell nucleus, where it drives a transcriptional program that is tailored to re-establish homeostasis. Using thin section electron microscopy, we found that yeast cells expand their ER volume at least 5-fold under UPR-inducing conditions. Surprisingly, we discovered that ER proliferation is accompanied by the formation of autophagosome-like structures that are densely and selectively packed with membrane stacks derived from the UPR-expanded ER. In analogy to pexophagy and mitophagy, which are autophagic processes that selectively sequester and degrade peroxisomes and mitochondria, the ER-specific autophagic process described utilizes several autophagy genes: they are induced by the UPR and are essential for the survival of cells subjected to severe ER stress. Intriguingly, cell survival does not require vacuolar proteases, indicating that ER sequestration into autophagosome-like structures, rather than their degradation, is the important step. Selective ER sequestration may help cells to maintain a new steady-state level of ER abundance even in the face of continuously accumulating unfolded proteins.
Conflict of interest statement
Competing interests. The authors have declared that no competing interests exist.
Figures

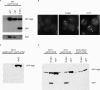
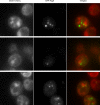

Comment in
-
Containing the damage of unfolded proteins.PLoS Biol. 2006 Dec;4(12):e442. doi: 10.1371/journal.pbio.0040442. Epub 2006 Nov 28. PLoS Biol. 2006. PMID: 20076517 Free PMC article. No abstract available.
References
-
- Wickner W, Schekman R. Protein translocation across biological membranes. Science. 2005;310:1452–1456. - PubMed
-
- Ellgaard L, Helenius A. Quality control in the endoplasmic reticulum. Nat Rev Mol Cell Biol. 2003;4:181–191. - PubMed
-
- McCracken AA, Brodsky JL. Recognition and delivery of ERAD substrates to the proteasome and alternative paths for cell survival. Curr Top Microbiol Immunol. 2005;300:17–40. - PubMed
-
- van Anken E, Braakman I. Versatility of the endoplasmic reticulum protein folding factory. Crit Rev Biochem Mol Biol. 2005;40:191–228. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

