Post-exposure prophylaxis for SIV revisited: animal model for HIV prevention
- PMID: 17132170
- PMCID: PMC1687192
- DOI: 10.1186/1742-6405-3-29
Post-exposure prophylaxis for SIV revisited: animal model for HIV prevention
Abstract
Background: A 4-week, uninterrupted treatment with 9-(2-phosphonyl-methoxypropyly)adenine (PMPA, commonly called tenofovir) completely prevents simian immunodeficiency virus (SIVmne) infection in cynomolgus macaques if treatment begins within 24 hours after SIVmne inoculation, but is less effective if treatment is delayed or duration of treatment is shortened. Critical factors for efficacy include timing and duration of treatment, potency of antiretroviral drug and a contribution from antiviral immune responses. Therefore, we evaluated the impact of one or more treatment interruptions plus SIVmne re-exposures on efficacy of PMPA treatment to prevent SIVmne infection in cynomolgus macaques. We also evaluated whether macaques with pre-existing SIV immune responses show increased efficacy of treatment. Eight PMPA-treated, virus-negative and seronegative macaques, and five PMPA-treated, virus-negative but weakly or strongly seropositive macaques were re-inoculated with SIVmne and treated with PMPA starting 24 hr post inoculation. Thereafter, they received either a 5-week treatment involving one interruption plus one SIVmne challenge or a 10-week treatment involving six interruptions plus six SIVmne challenges early during treatment. Parameters measured were plasma SIV RNA, SIV-antibody response, CD4+ T lymphocyte subsets and in vivo CD8+ cell-suppression of virus infection.
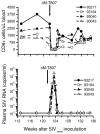
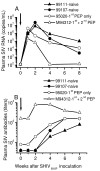
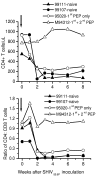
Results: All seronegative macaques developed persistent antibody response beginning 4 to 8 weeks after stopping PMPA-treatment in absence of viremia in a majority of macaques and coinciding with onset of intermittent viremia in other macaques. In contrast, all weakly or strongly seropositive macaques showed immediate increase in titers (> 1600) of SIV antibodies, even before the end of PMPA-treatment, and in absence of detectable viremia. However, in vivo CD8+-cell depletion revealed CD8 cell-suppression of viremia and persistence of virus in the macaques as long as 2 years after PMPA-treatment, even in aviremic macaques. Unlike untreated macaques, a treated macaque controlled viral replication and blocked CD4+ T cell depletion when challenged with a heterologous chimeric SIV/HIV-1 virus called SHIV89.6P.
Conclusion: A single interruption plus one SIVmne challenge was as sufficient as six interruptions plus six SIVmne challenges in reducing efficacy of PMPA, but results in long-term persistence of virus infection suppressed by CD8+ cells. Efficacy of PMPA treatment was highest in macaques with pre-existing SIV immune responses.
Figures


Similar articles
-
Effectiveness of postinoculation (R)-9-(2-phosphonylmethoxypropyl) adenine treatment for prevention of persistent simian immunodeficiency virus SIVmne infection depends critically on timing of initiation and duration of treatment.J Virol. 1998 May;72(5):4265-73. doi: 10.1128/JVI.72.5.4265-4273.1998. J Virol. 1998. PMID: 9557716 Free PMC article.
-
Administration of 9-[2-(phosphonomethoxy)propyl]adenine (PMPA) for prevention of perinatal simian immunodeficiency virus infection in rhesus macaques.AIDS Res Hum Retroviruses. 1998 Jun 10;14(9):761-73. doi: 10.1089/aid.1998.14.761. AIDS Res Hum Retroviruses. 1998. PMID: 9643376
-
Prevention of SIV rectal transmission and priming of T cell responses in macaques after local pre-exposure application of tenofovir gel.PLoS Med. 2008 Aug 5;5(8):e157; discussion e157. doi: 10.1371/journal.pmed.0050157. PLoS Med. 2008. PMID: 18684007 Free PMC article.
-
Transient early post-inoculation anti-retroviral treatment facilitates controlled infection with sparing of CD4+ T cells in gut-associated lymphoid tissues in SIVmac239-infected rhesus macaques, but not resistance to rechallenge.J Med Primatol. 2003 Aug;32(4-5):201-10. doi: 10.1034/j.1600-0684.2003.00026.x. J Med Primatol. 2003. PMID: 14498980
-
Two doses of PMPA protect newborn macaques against oral simian immunodeficiency virus infection.AIDS. 1998 Jun 18;12(9):F79-83. doi: 10.1097/00002030-199809000-00001. AIDS. 1998. PMID: 9662190
Cited by
-
Repeated DNA therapeutic vaccination of chronically SIV-infected macaques provides additional virological benefit.Vaccine. 2010 Feb 23;28(8):1962-74. doi: 10.1016/j.vaccine.2009.10.099. Vaccine. 2010. PMID: 20188252 Free PMC article.
-
Systemic administration of antiretrovirals prior to exposure prevents rectal and intravenous HIV-1 transmission in humanized BLT mice.PLoS One. 2010 Jan 21;5(1):e8829. doi: 10.1371/journal.pone.0008829. PLoS One. 2010. PMID: 20098623 Free PMC article.
-
Initiation, discontinuation, and restarting HIV pre-exposure prophylaxis: ongoing implementation strategies.Lancet HIV. 2020 Oct;7(10):e721-e730. doi: 10.1016/S2352-3018(20)30203-4. Epub 2020 Aug 27. Lancet HIV. 2020. PMID: 32861269 Free PMC article. Review.
-
Critical issues in mucosal immunity for HIV-1 vaccine development.J Allergy Clin Immunol. 2008 Jul;122(1):3-9; quiz 10-1. doi: 10.1016/j.jaci.2008.03.036. Epub 2008 May 12. J Allergy Clin Immunol. 2008. PMID: 18468671 Free PMC article. Review.
-
Effectiveness of islatravir post-exposure prophylaxis after intravenous challenge with simian immunodeficiency virus in rhesus macaques.J Int AIDS Soc. 2025 Jun;28(6):e26507. doi: 10.1002/jia2.26507. J Int AIDS Soc. 2025. PMID: 40534150 Free PMC article.
References
-
- WHO/UNAIDS Progress on Global Access to HIV Antiretroviral Therapy: A Report on "3 by 5" and Beyond. 2006.
-
- Patella Fj, Jr, Baker RK, Moorman AC, Chmiel JS, Wood KC, Brooks JT, Holmberg SD, and HIV Outpatient Study Investigators Mortality in the highly active antiretroviral therapy era: Changing causes of death and disease in the HIV outpatient study. J Acquir Immune Defic Syndr. 2006;43:27–34. doi: 10.1097/01.qai.0000233310.90484.16. - DOI - PubMed
-
- UNAIDS/WHO AIDS Epidemic Update: Special Report on HIV Prevention. 2005.
-
- Dorenbaum A, Cunningham CK, Gelber RD, Culnane M, Mofenson L, Britto P, Rekacewicz C, Newell ML, Delfraissy JF, Cunningham-Schrader B, Mirochnick M, International PACTG 316 Team Two-dose intrapartum/newborn nevirapine and standard antiretroviral therapy to reduce perinatal HIV transmission: a randomized trial. JAMA. 2002;288:189–198. doi: 10.1001/jama.288.2.189. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

