The effectiveness of a treatment protocol for male lower urinary tract symptoms in general practice: a practical randomised controlled trial
- PMID: 17132382
- PMCID: PMC1934054
The effectiveness of a treatment protocol for male lower urinary tract symptoms in general practice: a practical randomised controlled trial
Abstract
Background: Randomised controlled trials have shown the efficacy of several treatment modalities for lower urinary tract symptoms (LUTS) in selected populations. The effectiveness in daily practice has hardly been investigated, especially in primary care and is dependent on choices between all possible treatment options and best investigated in a comprehensive study, including all treatment modalities (watchful waiting, alpha-blockers, 5-alpha-reductase inhibitors, and surgery).
Aim: Assessment of the effectiveness of a comprehensive treatment protocol for LUTS in primary care.
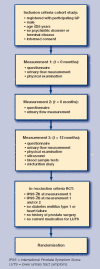
Design of study: Randomised controlled trial.
Setting: Fourteen general practices in the Netherlands.
Intervention: treatment protocol based on a formalised expert opinion. Control condition: usual care.
Study population: 208 subjects with moderate to severe LUTS (IPSS > or =8, median = 13).
Outcome measures: symptom severity (IPSS [International Prostate Symptom Score]), bother score (Dan-PSS [Danish Prostate Symptom Score]), and maximum urinary flow (Q(max)); incidence of acute urinary retention and urinary tract infections.
Results: In the intervention group markedly more subjects used an alpha-blocker at end of follow-up than in the usual care group (24% versus 6%). No significant differences were found between intervention and control group in IPSS, Q(max) or Dan-PSS.
Conclusion: alpha-blockers and watchful waiting are the most frequent treatment modalities for LUTS in primary care. Our study showed no evidence that a protocol using well-defined indications for all possible treatment modalities based on a formalised expert opinion procedure has added value. Based on our results, we cannot recommend a broadening of the indication for alpha-blockers, which, however, seems to be the current trend.
Figures
Similar articles
-
Influence of baseline variables on changes in International Prostate Symptom Score after combined therapy with dutasteride plus tamsulosin or either monotherapy in patients with benign prostatic hyperplasia and lower urinary tract symptoms: 4-year results of the CombAT study.BJU Int. 2014 Apr;113(4):623-35. doi: 10.1111/bju.12500. Epub 2014 Jan 9. BJU Int. 2014. PMID: 24127818 Clinical Trial.
-
Alfuzosin 10 mg once daily for treating benign prostatic hyperplasia: a 3-year experience in real-life practice.BJU Int. 2008 Apr;101(7):847-52. doi: 10.1111/j.1464-410X.2008.07458.x. BJU Int. 2008. PMID: 18321317
-
Monotherapy with tadalafil or tamsulosin similarly improved lower urinary tract symptoms suggestive of benign prostatic hyperplasia in an international, randomised, parallel, placebo-controlled clinical trial.Eur Urol. 2012 May;61(5):917-25. doi: 10.1016/j.eururo.2012.01.013. Epub 2012 Jan 20. Eur Urol. 2012. PMID: 22297243 Clinical Trial.
-
BPH progression: concept and key learning from MTOPS, ALTESS, COMBAT, and ALF-ONE.BJU Int. 2008 Mar;101 Suppl 3:17-21. doi: 10.1111/j.1464-410X.2008.07497.x. BJU Int. 2008. PMID: 18307681 Review.
-
Treatment of lower urinary tract symptoms suggestive of benign prostatic hyperplasia in relation to the patient's risk profile for progression.BJU Int. 2005 Jun;95 Suppl 4:6-11. doi: 10.1111/j.1464-410X.2005.05488.x. BJU Int. 2005. PMID: 15871730 Review.
Cited by
-
How usual is usual care in pragmatic intervention studies in primary care? An overview of recent trials.Br J Gen Pract. 2010 Jul;60(576):e305-18. doi: 10.3399/bjgp10X514819. Br J Gen Pract. 2010. PMID: 20594432 Free PMC article.
References
-
- Department of Health. Prescription cost analysis, England. 2003. http://www.dh.gov.uk/PublicationsAndStatistics/Publications/Publications... (accessed 1 Nov 2006)
-
- Bruskewitz R. Management of symptomatic BPH in the US: who is treated and how? Eur Urol. 1999;36(Suppl 3):7–13. - PubMed
-
- Wilt TJ, Howe RW, Rutks IR, MacDonald R. Terazosin for benign prostatic hyperplasia. Cochrane Database Syst Rev. 2002;(4) CD003851. - PubMed
-
- Wilt TJ, Mac Donald R, Rutks I. Tamsulosin for benign prostatic hyperplasia. Cochrane Database Syst Rev. 2003;(1) CD002081. - PubMed
-
- Roehrborn CG, Marks LS, Fenter TC, et al. Efficacy and safety of dutasteride in the four-year treatment of men with benign prostatic hyperplasia. Urology. 2004;63(4):709–715. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous