Anthrax pathogen evades the mammalian immune system through stealth siderophore production
- PMID: 17132740
- PMCID: PMC1693691
- DOI: 10.1073/pnas.0607055103
Anthrax pathogen evades the mammalian immune system through stealth siderophore production
Abstract
Systemic anthrax, caused by inhalation or ingestion of Bacillus anthracis spores, is characterized by rapid microbial growth stages that require iron. Tightly bound and highly regulated in a mammalian host, iron is scarce during an infection. To scavenge iron from its environment, B. anthracis synthesizes by independent pathways two small molecules, the siderophores bacillibactin (BB) and petrobactin (PB). Despite the great efficiency of BB at chelating iron, PB may be the only siderophore necessary to ensure full virulence of the pathogen. In the present work, we show that BB is specifically bound by siderocalin, a recently discovered innate immune protein that is part of an antibacterial iron-depletion defense. In contrast, neither PB nor its ferric complex is bound by siderocalin. Although BB incorporates the common 2,3-dihydroxybenzoyl iron-chelating subunit, PB is novel in that it incorporates the very unusual 3,4-dihydroxybenzoyl chelating subunit. This structural variation results in a large change in the shape of both the iron complex and the free siderophore that precludes siderocalin binding, a stealthy evasion of the immune system. Our results indicate that the blockade of bacterial siderophore-mediated iron acquisition by siderocalin is not restricted to enteric pathogenic organisms and may be a general defense mechanism against several different bacterial species. Significantly, to evade this innate immune response, B. anthracis produces PB, which plays a key role in virulence of the organism. This analysis argues for antianthrax strategies targeting siderophore synthesis and uptake.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

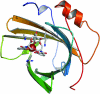
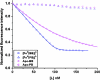

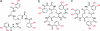
References
-
- Dixon TC, Meselson M, Guillemin J, Hanna PC. N Engl J Med. 1999;341:815–826. - PubMed
-
- Friedlander AM. Curr Clin Top Infect Dis. 2000;20:335–349. - PubMed
-
- Cendrowski S, MacArthur W, Hanna PC. Mol Microbiol. 2004;51:407–417. - PubMed
-
- Dertz EA, Raymond KN. In: Comprehensive Coordination Chemistry II. McCleverty J, Meyer T, editors. Vol 8. Oxford: Pergamon; 2003. pp. 141–168.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

