Complex relationship between BOLD signal and synchronization/desynchronization of human brain MEG oscillations
- PMID: 17133396
- PMCID: PMC6871384
- DOI: 10.1002/hbm.20322
Complex relationship between BOLD signal and synchronization/desynchronization of human brain MEG oscillations
Abstract
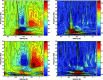
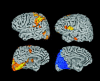
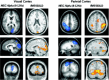
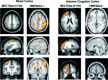
Functional magnetic resonance imaging (fMRI) depends on the coupling of cerebral blood flow, energy demand, and neural activity. The precise nature of this interaction, however, is poorly understood. A positive correlation between BOLD-response and cortically generated local field potentials, which reflect the weighted average of synchronized dentrosomatic components of pyramidal synaptic signals, has been demonstrated. Likewise, positive BOLD-responses have been reported in conjunction with scalp-recorded synchronized electromagnetic activity by a number of groups. However, it is not yet clear how the opposite electromagnetic pattern, i.e. cortical desynchronization, is related to the BOLD signal. To address this question, we conducted a combined event-related fMRI and 275 sensor whole-head MEG study during identical visual two-choice reaction time task conditions in 10 human subjects. We found complex sequences of MEG-synchronization and desynchronization across a wide frequency range in the visual and motor area in close correspondence with "locales" of positive BOLD-responses. These results indicate that a correspondence of positive BOLD-responses is not exclusively found for cortical synchronization but also for desynchronization, suggesting that the relationship between BOLD signals and electromagnetic activity might be more complex than previously thought.
(c) 2006 Wiley-Liss, Inc.
Figures



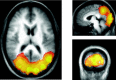
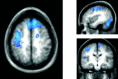
References
-
- Adjamian P, Holliday IE, Barnes GR, Hillebrand A, Hadjipapas A, Singh KD( 2004): Induced visual illusions and γ oscillations in human primary visual cortex. Eur J Neurosci 20: 587–592. - PubMed
-
- Aghakhani Y, Bagshaw AP, Benar CG, Hawco C, Andermann F, Dubeau F, Gotman J( 2004): fMRI activation during spike and wave discharges in idiopathic generalized epilepsy. Brain 127: 1127–1144. - PubMed
-
- Attwell D, Iadecola C ( 2002): The neural basis of functional brain imaging signals. Trends Neurosci 25: 621–625. - PubMed
-
- Başar E ( 1988): EEG‐dynamics and evoked potentials in sensory and cognitive processing by the brain In: Başar E,editor. Dynamics of Sensory and Cognitive Processing by the Brain. New York: Springer; pp 30–55.
-
- Benar CG, Gross DW, Wang Y, Petre V, Pike B, Dubeau F, Gotman J ( 2002): The BOLD response to interictal epileptiform discharges. Neuroimage 17: 1182–1192. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

