Chemical and biological aspects of Narcissus alkaloids
- PMID: 17133715
- PMCID: PMC7118783
- DOI: 10.1016/s1099-4831(06)63003-4
Chemical and biological aspects of Narcissus alkaloids
Abstract
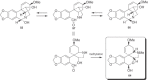
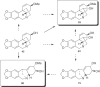
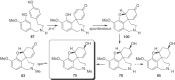
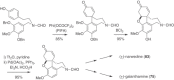
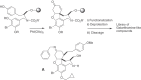
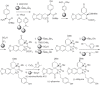
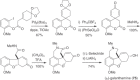
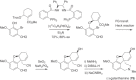
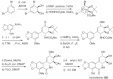
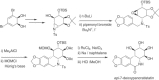
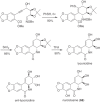
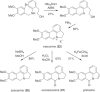
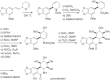
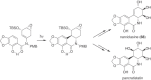
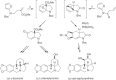
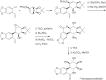
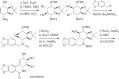
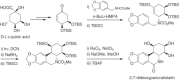
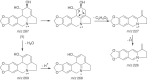
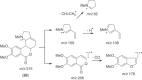
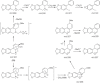
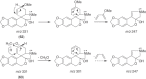
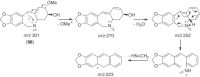
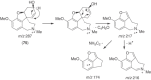
This chapter discusses the chemical and biological aspects of Narcissus alkaloids. Numerous alkaloids have been isolated from Narcissus speciesasaresult of the continuing search for novel alkaloids with pharmacological activity in the Amaryllidaceae family. The alkaloids isolated from this genus, classified in relation to the different skeleton types. The different Narcissus wild species and intersectional hybrids, grouped into subgenera and sections, with their corresponding alkaloids, arranged according to their ring system are listed. The biosynthetic pathways of Narcissus alkaloids includes: (1) enzymatic preparation of the precursors, (2) primary cyclization mechanisms, (3) enzymatic preparation of intermediates, (4) secondary cyclization, diversification, and restructuring. The chapter discusses proton nuclear magnetic resonance (1H NMR), carbon nuclear magnetic resonance (13C NMR), and mass spectrometry (MS) for Narcissus alkaloids. A list of the different Narcissus alkaloids, their spectroscopic properties, and literature with the most recent spectroscopic data is given. Several Narcissus extracts shows the following activities: antiviral, prophage induction, antibacterial, antifungal, antimalarial, insecticidal, cytotoxic, antitumor, antimitotic, antiplatelet, hypotensive, emetic, acetylcholine esterase inhibitory, antifertility, antinociceptive, chronotropic, pheromone, plant growth inhibitor, and allelopathic.
Figures
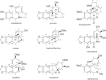
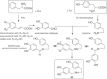
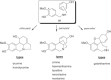
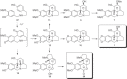
Similar articles
-
Solid-phase extraction and reversed-phase high-performance liquid chromatography of the five major alkaloids in Narcissus confusus.Phytochem Anal. 2002 Nov-Dec;13(6):311-5. doi: 10.1002/pca.660. Phytochem Anal. 2002. PMID: 12494748
-
Evolution of alkaloid biosynthesis in the genus Narcissus.Phytochemistry. 2014 Mar;99:95-106. doi: 10.1016/j.phytochem.2013.11.002. Epub 2014 Jan 23. Phytochemistry. 2014. PMID: 24461780
-
Seasonal accumulation of major alkaloids in organs of pharmaceutical crop Narcissus Carlton.Phytochemistry. 2013 Apr;88:43-53. doi: 10.1016/j.phytochem.2012.12.008. Epub 2013 Jan 11. Phytochemistry. 2013. PMID: 23318143
-
Lycopodium alkaloids--synthetic highlights and recent developments.Alkaloids Chem Biol. 2013;72:1-151. doi: 10.1016/b978-0-12-407774-4.00001-7. Alkaloids Chem Biol. 2013. PMID: 24712098 Review. No abstract available.
-
Advances on the Amaryllidacea Alkaloids Collected in South Africa, Andean South America and the Mediterranean Basin.Molecules. 2023 May 12;28(10):4055. doi: 10.3390/molecules28104055. Molecules. 2023. PMID: 37241796 Free PMC article. Review.
Cited by
-
Antiparasitic Activity of Hippeastrum Species and Synergistic Interaction between Montanine and Benznidazole against Trypanosoma cruzi.Microorganisms. 2023 Jan 6;11(1):144. doi: 10.3390/microorganisms11010144. Microorganisms. 2023. PMID: 36677436 Free PMC article.
-
Lycorine Carbamate Derivatives for Reversing P-glycoprotein-Mediated Multidrug Resistance in Human Colon Adenocarcinoma Cells.Int J Mol Sci. 2023 Jan 20;24(3):2061. doi: 10.3390/ijms24032061. Int J Mol Sci. 2023. PMID: 36768386 Free PMC article.
-
Chemical and Biological Aspects of Different Species of the Genus Clinanthus Herb. (Amaryllidaceae) from South America.Molecules. 2023 Jul 14;28(14):5408. doi: 10.3390/molecules28145408. Molecules. 2023. PMID: 37513280 Free PMC article.
-
Effectiveness of Narciclasine in Suppressing the Inflammatory Response in Sepsis: Molecular Docking and In Silico Studies.Bioinform Biol Insights. 2024 Mar 16;18:11779322241233436. doi: 10.1177/11779322241233436. eCollection 2024. Bioinform Biol Insights. 2024. PMID: 38495740 Free PMC article.
-
Alkaloids of Phaedranassa dubia (Kunth) J.F. Macbr. and Phaedranassa brevifolia Meerow (Amaryllidaceae) from Ecuador and its cholinesterase-inhibitory activity.S Afr J Bot. 2021 Jan;136:91-99. doi: 10.1016/j.sajb.2020.09.007. Epub 2020 Sep 18. S Afr J Bot. 2021. PMID: 32982003 Free PMC article.
References
-
- A. W. Meerow and D. A. Snijman, in “The Families and Genera of Vascular Plants” (K. Kubitzki, ed.), vol. III, p. 83. Springer, Berlin, 1998.
-
- Ito M., Kawamoto A., Kita Y., Yukawa T., Kurita S. J. Plant Res. 1999;112:207.
-
- G. R. Hanks, in “Narcissus and daffodil: the genus Narcissus” (G.R. Hanks, ed.), vol. 21 in the series: “Medicinal and Aromatic Plants – Industrial Profiles”, p. 1. Taylor & Francis, London, 2002.
-
- B. Mathew, in “Narcissus and daffodil: the genus Narcissus” (G.R. Hanks, ed.), vol. 21 in the series: “Medicinal and Aromatic Plants – Industrial Profiles”, p. 30. Taylor & Francis, London, 2002.
-
- Graham S.W., Barrett S.C.H. Am. J. Bot. 2004;91:1007. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
