Long term effects of bosentan treatment in adult patients with pulmonary arterial hypertension related to congenital heart disease (Eisenmenger physiology): safety, tolerability, clinical, and haemodynamic effect
- PMID: 17135220
- PMCID: PMC1955562
- DOI: 10.1136/hrt.2006.097360
Long term effects of bosentan treatment in adult patients with pulmonary arterial hypertension related to congenital heart disease (Eisenmenger physiology): safety, tolerability, clinical, and haemodynamic effect
Abstract
Background: Oral bosentan is an established treatment for pulmonary arterial hypertension (PAH).
Objective: To evaluate safety, tolerability, and clinical and haemodynamic effects of bosentan in patients with PAH related to congenital heart disease (CHD).
Patients: 22 patients with CHD related PAH (8 men, 14 women, mean (SD) age 38 (10) years) were treated with oral bosentan (62.5 mg x 2/day for the first 4 weeks and then 125 mg x 2/day).
Main outcome measures: Clinical status, liver enzymes, World Health Organisation (WHO) functional class, resting oxygen saturations and 6-min walk test (6MWT) were assessed at baseline and at 1, 3, 6, and 12 months. Haemodynamic evaluation with cardiac catheterisation was performed at baseline and at 12 month follow-up.
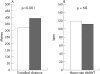
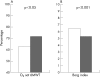
Results: 12 patients had ventricular septal defect, 5 atrioventricular canal, 4 single ventricle, and 1 atrial septal defect. All patients tolerated bosentan well. No major side effects were seen. After a year of treatment, an improvement was seen in WHO functional class (2.5 (0.7) v 3.1 (0.7); p<0.05), oxygen saturation at rest (87 (6%) v 81 (9); p<0.001), heart rate at rest (81 (10) v 87 (14) bpm; p<0.05), distance travelled in the 6MWT (394 (73) v 320 (108) m; p<0.001), oxygen saturation at the end of the 6MWT (71 (14) v 63 (17%); p<0.05), Borg index (5.3 (1.8) v 6.5 (1.3); p<0.001), pulmonary vascular resistances index (14 (9) v 22 (12) WU m(2); p<0.001), systemic vascular resistances index (23 (11) v 27 (10) WU.m(2); p<0.01), pulmonary vascular resistances index/systemic vascular resistances index (0.6 (0.5) v 0.9 (0.6); p<0.05); pulmonary (4.0 (1.3) v 2.8 (0.9) l/min/m2; p<0.001) and systemic cardiac output (4.2 (1.4) v 3.4 (1.1) l/min/m2; p<0.05).
Conclusions: Bosentan was safe and well tolerated in adults with CHD related PAH during 12 months of treatment. Clinical status, exercise tolerance, and pulmonary haemodynamics improved considerably.
Conflict of interest statement
Competing interests: None.
Similar articles
-
Bosentan-sildenafil association in patients with congenital heart disease-related pulmonary arterial hypertension and Eisenmenger physiology.Int J Cardiol. 2012 Mar 22;155(3):378-82. doi: 10.1016/j.ijcard.2010.10.051. Epub 2010 Nov 16. Int J Cardiol. 2012. PMID: 21081251 Clinical Trial.
-
Effect of the oral endothelin antagonist bosentan on the clinical, exercise, and haemodynamic status of patients with pulmonary arterial hypertension related to congenital heart disease.Heart. 2005 Nov;91(11):1447-52. doi: 10.1136/hrt.2004.051961. Epub 2005 Mar 10. Heart. 2005. PMID: 15761050 Free PMC article. Clinical Trial.
-
Long-term oral bosentan treatment in patients with pulmonary arterial hypertension related to congenital heart disease: a 2-year study.Heart. 2007 Mar;93(3):350-4. doi: 10.1136/hrt.2006.100388. Epub 2006 Sep 15. Heart. 2007. PMID: 16980516 Free PMC article. Clinical Trial.
-
Safety and tolerability evaluation of oral bosentan in adult congenital heart disease associated pulmonary arterial hypertension: a systematic review and meta-analysis.Eur Rev Med Pharmacol Sci. 2014;18(5):638-45. Eur Rev Med Pharmacol Sci. 2014. PMID: 24668703
-
What is the position of pulmonary arterial hypertension-specific drug therapy in patients with Eisenmenger syndrome: A systematic review and meta-analysis.Medicine (Baltimore). 2019 May;98(20):e15632. doi: 10.1097/MD.0000000000015632. Medicine (Baltimore). 2019. PMID: 31096477 Free PMC article.
Cited by
-
Pulmonary arterial hypertension: a comparison between children and adults.Eur Respir J. 2011 Mar;37(3):665-77. doi: 10.1183/09031936.00056110. Eur Respir J. 2011. PMID: 21357924 Free PMC article. Review.
-
Adult patients with pulmonary arterial hypertension due to congenital heart disease: a review on advanced medical treatment with bosentan.Ther Clin Risk Manag. 2010 Sep 7;6:359-66. doi: 10.2147/tcrm.s8397. Ther Clin Risk Manag. 2010. PMID: 20856682 Free PMC article.
-
Long-term results of treatment with bosentan in adult Eisenmenger's syndrome patients with Down's syndrome related to congenital heart disease.BMC Cardiovasc Disord. 2013 Sep 18;13:74. doi: 10.1186/1471-2261-13-74. BMC Cardiovasc Disord. 2013. PMID: 24047157 Free PMC article.
-
Analysis of endothelin-1 and endothelin-1 receptor A gene polymorphisms in patients with pulmonary arterial hypertension.Intern Emerg Med. 2012 Oct;7(5):425-30. doi: 10.1007/s11739-011-0643-2. Epub 2011 Jul 20. Intern Emerg Med. 2012. PMID: 21773759
-
Current Management and Future Directions for Pulmonary Arterial Hypertension Associated with Congenital Heart Disease.J Pers Med. 2023 Dec 20;14(1):5. doi: 10.3390/jpm14010005. J Pers Med. 2023. PMID: 38276220 Free PMC article. Review.
References
-
- Farber H W, Loscalzo J. Pulmonary arterial hypertension. N Engl J Med 20043511655–1665. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical