The assembly pathway of the 19S regulatory particle of the yeast 26S proteasome
- PMID: 17135287
- PMCID: PMC1783769
- DOI: 10.1091/mbc.e06-07-0635
The assembly pathway of the 19S regulatory particle of the yeast 26S proteasome
Abstract
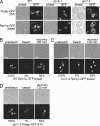
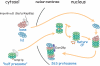
The 26S proteasome consists of the 20S proteasome (core particle) and the 19S regulatory particle made of the base and lid substructures, and it is mainly localized in the nucleus in yeast. To examine how and where this huge enzyme complex is assembled, we performed biochemical and microscopic characterization of proteasomes produced in two lid mutants, rpn5-1 and rpn7-3, and a base mutant DeltaN rpn2, of the yeast Saccharomyces cerevisiae. We found that, although lid formation was abolished in rpn5-1 mutant cells at the restrictive temperature, an apparently intact base was produced and localized in the nucleus. In contrast, in DeltaN rpn2 cells, a free lid was formed and localized in the nucleus even at the restrictive temperature. These results indicate that the modules of the 26S proteasome, namely, the core particle, base, and lid, can be formed and imported into the nucleus independently of each other. Based on these observations, we propose a model for the assembly process of the yeast 26S proteasome.
Figures






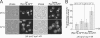
References
-
- Bachmair A., Finley D., Varshavsky A. In vivo half-life of a protein is a function of its amino-terminal residue. Science. 1986;234:179–186. - PubMed
-
- Burk D., Dawson D., Stearns T. Methods in Yeast Genetics. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 2000.
-
- Cadwell R. C., Joyce G. F. Randomization of genes by PCR mutagenesis. PCR Methods Appl. 1992;2:28–33. - PubMed
-
- Chen P., Hochstrasser M. Autocatalytic subunit processing couples active site formation in the 20S proteasome to completion of assembly. Cell. 1996;86:961–972. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

