Aberrant translation of cytochrome c oxidase subunit 1 mRNA species in the absence of Mss51p in the yeast Saccharomyces cerevisiae
- PMID: 17135289
- PMCID: PMC1783774
- DOI: 10.1091/mbc.e06-09-0803
Aberrant translation of cytochrome c oxidase subunit 1 mRNA species in the absence of Mss51p in the yeast Saccharomyces cerevisiae
Abstract
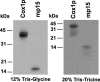
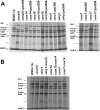
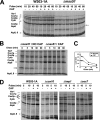
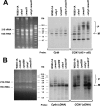
Expression of yeast mitochondrial genes depends on specific translational activators acting on the 5'-untranslated region of their target mRNAs. Mss51p is a translational factor for cytochrome c oxidase subunit 1 (COX1) mRNA and a key player in down-regulating Cox1p expression when subunits with which it normally interacts are not available. Mss51p probably acts on the 5'-untranslated region of COX1 mRNA to initiate translation and on the coding sequence itself to facilitate elongation. Mss51p binds newly synthesized Cox1p, an interaction that could be necessary for translation. To gain insight into the different roles of Mss51p on Cox1p biogenesis, we have analyzed the properties of a new mitochondrial protein, mp15, which is synthesized in mss51 mutants and in cytochrome oxidase mutants in which Cox1p translation is suppressed. The mp15 polypeptide is not detected in cox14 mutants that express Cox1p normally. We show that mp15 is a truncated translation product of COX1 mRNA whose synthesis requires the COX1 mRNA-specific translational activator Pet309p. These results support a key role for Mss51p in translationally regulating Cox1p synthesis by the status of cytochrome oxidase assembly.
Figures






References
-
- Arlt H., Tauer R., Feldmann H., Neupert W., Langer T. The YTA10-12 complex, an AAA protease with chaperone-like activity in the inner membrane of mitochondria. Cell. 1996;85:875–885. - PubMed
-
- Ausubel F. M., Brent R., Kingston R. E., Moore D. D., Seidman J. G., Smith J. A., Struhl K. Current Protocols in Molecular Biology. Vol. 2. New York: Wiley; 1994. Saccharomyces cerevisiae; p. 13.
-
- Barrientos A., Barros M. H., Valnot I., Rotig A., Rustin P., Tzagoloff A. Cytochrome oxidase in health and disease. Gene. 2002a;286:53–63. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

