Allelic deletion of the MEN1 gene in duodenal gastrin and somatostatin cell neoplasms and their precursor lesions
- PMID: 17135306
- PMCID: PMC1942169
- DOI: 10.1136/gut.2006.108910
Allelic deletion of the MEN1 gene in duodenal gastrin and somatostatin cell neoplasms and their precursor lesions
Abstract
Background: Patients with a multiple endocrine neoplasia type 1 (MEN1)-associated Zollinger-Ellison syndrome (ZES) show multifocal duodenal gastrinomas and precursor lesions.
Aims: To test these lesions for loss of heterozygosity (LOH) of the MEN1 gene locus on chromosome 11q13, and to investigate whether the MEN1-related endocrine cell changes also involved somatostatin cells.
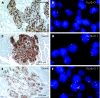
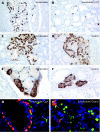
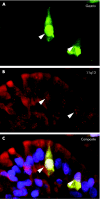
Material and methods: Tissue specimens from six patients with MEN1 and ZES were analysed by immunohistochemistry and immunofluorescence. LOH analysis was performed by fluorescence in situ hybridisation (FISH), using probes containing the MEN1 gene locus and the centromere 11 (C11) region. For simultaneous analysis of hormones and allelic deletions, a combined FISH/immunofluorescence protocol was established.
Results: 28 of a total of 33 duodenal neuroendocrine tumours (NETs) were gastrin-producing tumours; 13/28 (46.4%) revealed LOH on 11q13 and/or C11. Five of the NETs were somatostatin-expressing tumours, two revealing LOH. Allelic loss was detected in tumours as small as 300 microm (gastrin) and 400 microm (somatostatin) in diameter. The gastrin-producing tumours showed different deletion/retention patterns. Hyperplastic somatostatin cell lesions, similar to those of the gastrin cells, were present in all patients. The hyperplastic lesions of both cell lines consistently retained both 11q13 alleles.
Conclusions: Allelic deletion of the MEN1 gene may reflect a pivotal event in the development of multifocal gastrin and somatostatin cell neoplasms in the duodenum of patients with MEN1. The observation of distinct deletion patterns in small synchronous tumours supports the concept that each gastrin-producing tumour in an individual MEN1 patient arises from an independent cell clone.
Conflict of interest statement
Competing interests: None.
Comment in
-
Pathogenesis of gastrinomas associated with multiple endocrine neoplasia type 1.Gut. 2007 May;56(5):606-7. doi: 10.1136/gut.2006.113985. Gut. 2007. PMID: 17440178 Free PMC article.
Similar articles
-
[Gastrin cell hyperplasia associated with duodenal MEN1-related gastrinomas: histopathology and genetics].Verh Dtsch Ges Pathol. 2007;91:320-9. Verh Dtsch Ges Pathol. 2007. PMID: 18314630 German.
-
Precursor lesions in patients with multiple endocrine neoplasia type 1-associated duodenal gastrinomas.Gastroenterology. 2005 May;128(5):1187-98. doi: 10.1053/j.gastro.2005.01.058. Gastroenterology. 2005. PMID: 15887103
-
Sporadic versus hereditary gastrinomas of the duodenum and pancreas: distinct clinico-pathological and epidemiological features.World J Gastroenterol. 2006 Sep 14;12(34):5440-6. doi: 10.3748/wjg.v12.i34.5440. World J Gastroenterol. 2006. PMID: 17006979 Free PMC article. Review.
-
Allelic deletions on chromosome 11q13 in multiple endocrine neoplasia type 1-associated and sporadic gastrinomas and pancreatic endocrine tumors.Cancer Res. 1997 Jun 1;57(11):2238-43. Cancer Res. 1997. PMID: 9187127
-
Endocrine precursor lesions and microadenomas of the duodenum and pancreas with and without MEN1: criteria, molecular concepts and clinical significance.Pathobiology. 2007;74(5):279-84. doi: 10.1159/000105810. Pathobiology. 2007. PMID: 17890894 Review.
Cited by
-
Pancreatic neuroendocrine tumors: biology, diagnosis,and treatment.Chin J Cancer. 2013 Jun;32(6):312-24. doi: 10.5732/cjc.012.10295. Epub 2012 Dec 14. Chin J Cancer. 2013. PMID: 23237225 Free PMC article. Review.
-
Gastrin: From Physiology to Gastrointestinal Malignancies.Function (Oxf). 2021 Nov 26;3(1):zqab062. doi: 10.1093/function/zqab062. eCollection 2022. Function (Oxf). 2021. PMID: 35330921 Free PMC article. Review.
-
Somatostatin stimulates menin gene expression by inhibiting protein kinase A.Am J Physiol Gastrointest Liver Physiol. 2008 Oct;295(4):G843-54. doi: 10.1152/ajpgi.00607.2007. Epub 2008 Aug 28. Am J Physiol Gastrointest Liver Physiol. 2008. PMID: 18755809 Free PMC article.
-
Gastrin Induces Nuclear Export and Proteasome Degradation of Menin in Enteric Glial Cells.Gastroenterology. 2017 Dec;153(6):1555-1567.e15. doi: 10.1053/j.gastro.2017.08.038. Epub 2017 Aug 30. Gastroenterology. 2017. PMID: 28859856 Free PMC article.
-
[Neuroendocrine neoplasms : Two families with distinct features unified in one classification (German version)].Pathologe. 2019 May;40(3):211-219. doi: 10.1007/s00292-019-0594-3. Pathologe. 2019. PMID: 30969346 German.
References
-
- Calender A, Morrison C D, Komminoth P.et al Multiple endocrine neoplasia type 1. In: DeLellis RA, Lloyd RV, Heitz PU, Eng C, eds. Pathology and genetic: tumours of endocrine organs. WHO classification of tumors Lyon: IARC Press, 2004218–227.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical