A synthetic phage lambda regulatory circuit
- PMID: 17135356
- PMCID: PMC1748174
- DOI: 10.1073/pnas.0603052103
A synthetic phage lambda regulatory circuit
Abstract
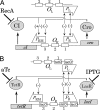
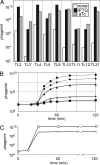
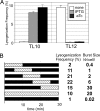
Analysis of synthetic gene regulatory circuits can provide insight into circuit behavior and evolution. An alternative approach is to modify a naturally occurring circuit, by using genetic methods to select functional circuits and evolve their properties. We have applied this approach to the circuitry of phage lambda. This phage grows lytically, forms stable lysogens, and can switch from this regulatory state to lytic growth. Genetic selections are available for each behavior. We previously replaced lambda Cro in the intact phage with a module including Lac repressor, whose function is tunable with small molecules, and several cis-acting sites. Here, we have in addition replaced lambda CI repressor with another tunable module, Tet repressor and several cis-acting sites. Tet repressor lacks several important properties of CI, including positive autoregulation and cooperative DNA binding. Using a combinatorial approach, we isolated phage variants with behavior similar to that of WT lambda. These variants grew lytically and formed stable lysogens. Lysogens underwent prophage induction upon addition of a ligand that weakens binding by the Tet repressor. Strikingly, however, addition of a ligand that weakens binding by Lac repressor also induced lysogens. This finding indicates that Lac repressor was present in the lysogens and was necessary for stable lysogeny. Therefore, these isolates had an altered wiring diagram from that of lambda. We speculate that this complexity is needed to compensate for the missing features. Our method is generally useful for making customized gene regulatory circuits whose activity is regulated by small molecules or protein cofactors.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

