Transplantation of human neural stem cells exerts neuroprotection in a rat model of Parkinson's disease
- PMID: 17135412
- PMCID: PMC6674904
- DOI: 10.1523/JNEUROSCI.3719-06.2006
Transplantation of human neural stem cells exerts neuroprotection in a rat model of Parkinson's disease
Abstract
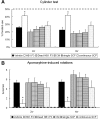
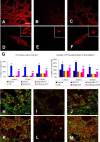
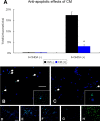
Neural stem cells (NSCs) possess high potencies of self-renewal and neuronal differentiation. We explored here whether transplantation of human NSCs cloned by v-myc gene transfer, HB1.F3 cells, is a feasible therapeutic option for Parkinson's disease. In vivo, green fluorescent protein-labeled HB1.F3 cells (200,000 viable cells in 3 microl of PBS) when stereotaxically transplanted (same-day lesion-transplant paradigm) into the 6-hydroxydopamine-lesioned striatum of rats significantly ameliorated parkinsonian behavioral symptoms compared with controls (vehicle, single bolus, or continuous minipump infusion of trophic factor, or killed cell grafts). Such graft-derived functional effects were accompanied by preservation of tyrosine hydroxylase (TH) immunoreactivity along the nigrostriatal pathway. Grafted HB1.F3 cells survived in the lesioned brain with some labeled with neuronal marker mitogen-activated protein 2 and decorated with synaptophysin-positive terminals. Furthermore, endogenous neurogenesis was activated in the subventricular zone of transplanted rats. To further explore the neuroprotective mechanisms underlying HB1.F3 cell transplantation, we performed cell culture studies and found that a modest number of HB1.F3 cells were TH and dopamine and cAMP-regulated phosphoprotein 32 positive, although most cells were nestin positive, suggesting a mixed population of mature and immature cells. Administration of the HB1.F3 supernatant to human derived dopaminergic SH-SY5Y cells and fetal rat ventral mesencephalic dopaminergic neurons protected against 6-hydroxydopamine neurotoxicity by suppressing apoptosis through Bcl-2 upregulation, which was blocked by anti-stem cell factor antibody alone, the phosphatidylinositol 3-kinase/Akt inhibitor LY294002 [2-(4-morpholinyl)-8-phenyl-1(4H)-benzopyran-4-one] alone, or a combination of both. These results suggest that HB1.F3 cell transplantation exerts neuroprotective effects against dopaminergic depletion in vitro and in vivo because of trophic factor secretion and neuronal differentiation.
Figures






References
-
- Alexi T, Borlongan CV, Faull RL, Williams CE, Clark RG, Gluckman PD, Hughes PE. Neuroprotective strategies for basal ganglia degeneration: Parkinson's and Huntington's diseases. Prog Neurobiol. 2000;60:409–470. - PubMed
-
- Arenas E. Stem cells in the treatment of Parkinson's disease. Brain Res Bull. 2002;57:795–808. - PubMed
-
- Armstrong RJ, Hurelbrink CB, Tyers P, Ratcliffe EL, Richards A, Dunnett SB, Rosser AE, Barker RA. The potential for circuit reconstruction by expanded neural precursor cells explored through porcine xenografts in a rat model of Parkinson's disease. Exp Neurol. 2002;175:98–111. - PubMed
-
- Ashman LK. The biology of stem cell factor and its receptor C-kit. Int J Biochem Cell Biol. 1999;31:1037–1051. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
