Neurocytoma is a tumor of adult neuronal progenitor cells
- PMID: 17135416
- PMCID: PMC6674894
- DOI: 10.1523/JNEUROSCI.0829-06.2006
Neurocytoma is a tumor of adult neuronal progenitor cells
Abstract
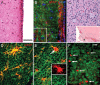
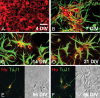
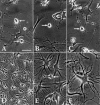
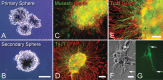
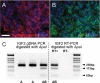
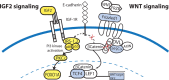
Central neurocytoma (CN) is a rare periventricular tumor, whose derivation, lineage potential, and molecular regulation have been mostly unexplored. We noted that CN cells exhibited an antigenic profile typical of neuronal progenitor cells in vivo, yet in vitro generated neurospheres, divided in response to bFGF (basic fibroblast growth factor), activated the neuroepithelial enhancer of the nestin gene, and gave rise to both neuron-like cells and astrocytes. When CN gene expression was compared with that of both normal adult VZ (ventricular zone) and E/nestin:GFP (green fluorescent protein)-sorted native neuronal progenitors, significant overlap was noted. Marker analysis suggested that the gene expression pattern of CN was that of a proneuronal population; glial markers were conspicuously absent, suggesting that the emergence of astroglia from CN occurred only with passage. The expression pattern of CN was distinguished from that of native progenitor cells by a cohort of differentially expressed genes potentially involved in both the oncogenesis and phenotypic restriction of neurocytoma. These included both IGF2 and several components of its signaling pathway, whose sharp overexpression implicated dysregulated autocrine IGF2 signaling in CN oncogenesis. Both receptors and effectors of canonical wnt signaling, as well as GDF8 (growth differentiation factor 8), PDGF-D, and neuregulin, were differentially overexpressed by CN, suggesting that CN is characterized by the concurrent overactivation of these pathways, which may serve to drive neurocytoma expansion while restricting tumor progenitor phenotype. This strategy of comparing the gene expression of tumor cells to that of the purified native progenitors from which they derive may provide a focused approach to identifying transcripts important to stem and progenitor cell oncogenesis.
Figures
References
-
- Akimoto J, Itoh H, Itoh Y, Miwa T. Histogenesis of central neurocytoma: an immunohistochemical and electronmicroscopic study of four cases. No Shinkei Geka. 1995;23:1083–1091. - PubMed
-
- Arsenijevic Y, Villemure JG, Brunet JF, Bloch JJ, Deglon N, Kostic C, Zurn A, Aebischer P. Isolation of multipotent neural precursors residing in the cortex of the adult human brain. Exp Neurol. 2001;170:48–62. - PubMed
-
- Bansal R, Lakhina V, Remedios R, Tole S. Expression of FGF receptors 1, 2, 3 in the embryonic and postnatal mouse brain compared with Pdgfralpha, Olig2 and Plp/dm20: implications for oligodendrocyte development. Dev Neurosci. 2003;25:83–95. - PubMed
-
- Barami K, Iversen K, Furneaux H, Goldman SA. Hu protein as an early marker of neuronal phenotypic differentiation by subependymal zone cells of the adult songbird forebrain. J Neurobiol. 1995;28:82–101. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
