Physiological and anatomical evidence for multisensory interactions in auditory cortex
- PMID: 17135481
- PMCID: PMC7116518
- DOI: 10.1093/cercor/bhl128
Physiological and anatomical evidence for multisensory interactions in auditory cortex
Abstract
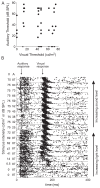
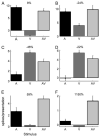
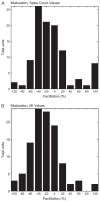
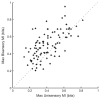
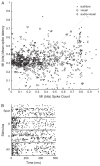
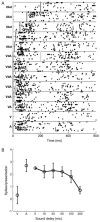
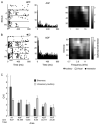
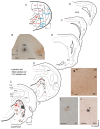
Recent studies, conducted almost exclusively in primates, have shown that several cortical areas usually associated with modality-specific sensory processing are subject to influences from other senses. Here we demonstrate using single-unit recordings and estimates of mutual information that visual stimuli can influence the activity of units in the auditory cortex of anesthetized ferrets. In many cases, these units were also acoustically responsive and frequently transmitted more information in their spike discharge patterns in response to paired visual-auditory stimulation than when either modality was presented by itself. For each stimulus, this information was conveyed by a combination of spike count and spike timing. Even in primary auditory areas (primary auditory cortex [A1] and anterior auditory field [AAF]), approximately 15% of recorded units were found to have nonauditory input. This proportion increased in the higher level fields that lie ventral to A1/AAF and was highest in the anterior ventral field, where nearly 50% of the units were found to be responsive to visual stimuli only and a further quarter to both visual and auditory stimuli. Within each field, the pure-tone response properties of neurons sensitive to visual stimuli did not differ in any systematic way from those of visually unresponsive neurons. Neural tracer injections revealed direct inputs from visual cortex into auditory cortex, indicating a potential source of origin for the visual responses. Primary visual cortex projects sparsely to A1, whereas higher visual areas innervate auditory areas in a field-specific manner. These data indicate that multisensory convergence and integration are features common to all auditory cortical areas but are especially prevalent in higher areas.
Conflict of interest statement
Figures
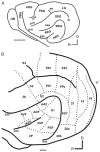

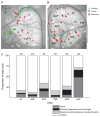
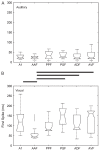
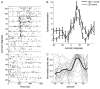

References
-
- Adams JC. Heavy metal intensification of DAB-based HRP reaction product. J Histochem Cytochem. 1981;29:775. - PubMed
-
- Aitkin LM, Kenyon CE, Philpott P. The representation of the auditory and somatosensory systems in the external nucleus of the cat inferior colliculus. J Comp Neurol. 1981;196:25–40. - PubMed
-
- Alitto HJ, Usrey WM. Influence of contrast on orientation and temporal frequency tuning in ferret primary visual cortex. J Neuro-physiol. 2004;91:2797–2808. - PubMed
-
- Bell AH, Corneil BD, Munoz DP, Meredith MA. Engagement of visual fixation suppresses sensory responsiveness and multisensory integration in the primate superior colliculus. Eur J Neurosci. 2003;18:2867–2873. - PubMed

