Hydrogen peroxide stimulates macrophages and monocytes to actively release HMGB1
- PMID: 17135572
- PMCID: PMC1808495
- DOI: 10.1189/jlb.0806540
Hydrogen peroxide stimulates macrophages and monocytes to actively release HMGB1
Abstract
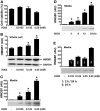
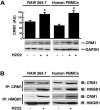
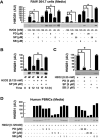
High mobility group box 1 (HMGB1) can be actively secreted by macrophages/monocytes in response to exogenous and endogenous inflammatory stimuli (such as bacterial endotoxin, TNF-alpha, IL-1, and IFN-gamma) or passively released by necrotic cells and mediates innate and adaptive inflammatory responses to infection and injury. Here, we demonstrated that a reactive oxygen species, hydrogen peroxide (H(2)O(2)), induces active and passive HMGB1 release from macrophage and monocyte cultures in a time- and dose-dependent manner. At nontoxic doses (e.g., 0.0125-0.125 mM), H(2)O(2) induced HMGB1 cytoplasmic translocation and active release within 3-24 h. At higher concentrations (e.g., 0.25 mM), however, H(2)O(2) exhibited cytotoxicity to macrophage and monocyte cell cultures and consequently, triggered active and passive HMGB1 release. In addition, H(2)O(2) stimulated potential interaction of HMGB1 with a nuclear export factor, chromosome region maintenance (CRM1), in macrophage/monocyte cultures. Inhibitors specific for the JNK (SP600125) and MEK (PD98059), but not p38 MAPK (SB203580), abrogated H(2)O(2)-induced, active HMGB1 release. Together, these data establish an important role for oxidative stress in inducing active HMGB1 release, potentially through a MAPK- and CRM1-dependent mechanism.
Figures

References
-
- Bergamini CM, Gambetti S, Dondi A, Cervellati C. Oxygen, reactive oxygen species and tissue damage. Curr. Pharm. Des. 2004;10:1611–1626. - PubMed
-
- Pouyssegur J. Signal transduction. An arresting start for MAPK. Science. 2000;290:1515–1518. - PubMed
-
- Kamata H, Honda S, Maeda S, Chang L, Hirata H, Karin M. Reactive oxygen species promote TNFα-induced death and sustained JNK activation by inhibiting MAP kinase phosphatases. Cell. 2005;120:649–661. - PubMed
-
- Handley ME, Thakker M, Pollara G, Chain BM, Katz DR. JNK activation limits dendritic cell maturation in response to reactive oxygen species by the induction of apoptosis. Free Radic. Biol. Med. 2005;38:1637–1652. - PubMed
-
- Wang H, Bloom O, Zhang M, Vishnubhakat JM, Ombrellino M, Che J, Frazier A, Yang H, Ivanova S, Borovikova L, Manogue KR, Faist E, Abraham E, Andersson J, Andersson U, Molina PE, Abumrad NN, Sama A, Tracey KJ. HMG-1 as a late mediator of endotoxin lethality in mice. Science. 1999;285:248–251. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

