Treatment for Adolescents with Depression Study (TADS): safety results
- PMID: 17135989
- PMCID: PMC3285253
- DOI: 10.1097/01.chi.0000240840.63737.1d
Treatment for Adolescents with Depression Study (TADS): safety results
Abstract
Objective: To compare the rates of physical, psychiatric, and suicide-related events in adolescents with MDD treated with fluoxetine alone (FLX), cognitive-behavioral therapy (CBT), combination treatment (COMB), or placebo (PBO).
Method: Safety assessments included adverse events (AEs) collected by spontaneous report, as well as systematic measures for specific physical and psychiatric symptoms. Suicidal ideation and suicidal behavior were systematically assessed by self- and clinician reports. Suicidal events were also reanalyzed by the Columbia Group and expert raters using the Columbia-Classification Algorithm for Suicidal Assessment used in the U.S. Food and Drug Administration reclassification effort.
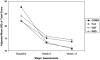
Results: Depressed adolescents reported high rates of physical symptoms at baseline, which improved as depression improved. Sedation, insomnia, vomiting, and upper abdominal pain occurred in at least 2% of those treated with FLX and/or COMB and at twice the rate of placebo. The rate of psychiatric AEs was 11% in FLX, 5.6% in COMB, 4.5% in PBO, and 0.9% in CBT. Suicidal ideation improved overall, with greatest improvement in COMB. Twenty-four suicide-related events occurred during the 12-week period: 5 patients (4.7%) in COMB, 10 (9.2%) in FLX, 5 (4.5%) in CBT, and 3 (2.7%) in placebo. Statistically, only FLX had more suicide-related events than PBO (p =.0402, odds ratio (OR) = 3.7, 95% CI 1.00-63.7). Only five actual attempts occurred (2 COMB, 2 FLX, 1 CBT, 0 PBO). There were no suicide completions.
Conclusions: Different methods for eliciting AEs produce different results. In general, as depression improves, physical complaints and suicidal ideation decrease in proportion to treatment benefit. In this study, psychiatric AEs and suicide-related events are more common in FLX-treated patients. COMB treatment may offer a more favorable safety profile than medication alone in adolescent depression.
Figures
Comment in
-
After TADS, can we measure up, catch up, and ante up?J Am Acad Child Adolesc Psychiatry. 2006 Dec;45(12):1456-60. doi: 10.1097/01.chi.0000237712.81378.9d. J Am Acad Child Adolesc Psychiatry. 2006. PMID: 17135990 No abstract available.
-
Glad for what TADS adds, but many TADS grads still sad.J Am Acad Child Adolesc Psychiatry. 2006 Dec;45(12):1461-4. doi: 10.1097/01.chi.0000237708.28013.2a. J Am Acad Child Adolesc Psychiatry. 2006. PMID: 17135991 No abstract available.
-
Cognitive behavioural therapy plus fluoxetine offers some safety advantages over fluoxetine alone in adolescents with depression.Evid Based Ment Health. 2007 Aug;10(3):85. doi: 10.1136/ebmh.10.3.85. Evid Based Ment Health. 2007. PMID: 17652567 No abstract available.
References
-
- Emslie GJ, Rush AJ, Weinberg WA, et al. A double-blind, randomized, placebo-controlled study of fluoxetine in depressed children and adolescents. Arch Gen Psychiatry. 1997;54:1031–1037. - PubMed
-
- Emslie GJ, Heiligenstein JH, Wagner KD, et al. Fluoxetine for acute treatment of depression in children and adolescents: a placebo-controlled, randomized clinical trial. J Am Acad Child and Adolesc Psychiatry. 2002;41:1205–1215. - PubMed
-
- Gibbons RD, Hur K, Bhaumik DK, Mann JJ. The relationship between antidepressant medication use and rate of suicide. Arch Gen Psychiatry. 2005;62:165–172. - PubMed
-
- Gray D, Achilles J, Keller T, et al. Utah youth suicide study, phase I: government agency contact before death. J Am Acad Child Adolesc Psychiatry. 2002;41:427–434. - PubMed
-
- Greenhill L, Vitiello B, Riddle M, et al. Review of safety assessment methods used in pediatric psychopharmacology. J Am Acad Child Adolesc Psychiatry. 2003;42:627–633. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical