Genome dynamics and transcriptional deregulation in aging
- PMID: 17137723
- PMCID: PMC1905494
- DOI: 10.1016/j.neuroscience.2006.09.060
Genome dynamics and transcriptional deregulation in aging
Abstract
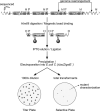
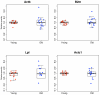
Genome instability has been implicated as a major cause of both cancer and aging. Using a lacZ-plasmid transgenic mouse model we have shown that mutations accumulate with age in a tissue-specific manner. Genome rearrangements, including translocations and large deletions, are a major component of the mutation spectrum in some tissues at old age such as heart. Such large mutations were also induced by hydrogen peroxide (H2O2) in lacZ-plasmid mouse embryonic fibroblasts (MEFs) and demonstrated to be replication-independent. This was in contrast to ultraviolet light-induced point mutations, which were much more abundant in proliferating than in quiescent MEFs. To test if large rearrangements could adversely affect patterns of gene expression we PCR-amplified global mRNA content of single MEFs treated with H2O2. Such treatment resulted in a significant increase in cell-to-cell variation in gene expression, which was found to parallel the induction and persistence of genome rearrangement mutations at the lacZ reporter locus. Increased transcriptional noise was also found among single cardiomyocytes from old mice as compared with similar cells from young mice. While these results do not directly indicate a cause and effect relationship between genome rearrangement mutations and transcriptional deregulation, they do underscore the stochastic nature of genotoxic effects on cells and tissues and could provide a mechanism for age-related cellular degeneration in postmitotic tissue, such as heart or brain.
Figures




References
-
- Bahar R, Hartmann CH, Rodriguez KA, Denny AD, Busuttil RA, Dolle ME, Calder RB, Chisholm GB, Pollock BH, Klein CA, Vijg J. Increased cell-to-cell variation in gene expression in ageing mouse heart. Nature. 2006;441:1011–1014. - PubMed
-
- Bailey KJ, Maslov AY, Pruitt SC. Accumulation of mutations and somatic selection in aging neural stem/progenitor cells. Aging Cell. 2004;3:391–397. - PubMed
-
- Becskei A, Kaufmann BB, van Oudenaarden A. Contributions of low molecule number and chromosomal positioning to stochastic gene expression. Nat Genet. 2005;37:937–944. - PubMed
-
- Boerrigter ME, Dolle ME, Martus HJ, Gossen JA, Vijg J. Plasmid-based transgenic mouse model for studying in vivo mutations. Nature. 1995;377:657–659. - PubMed
-
- Busuttil RA, Garcia AM, Cabrera C, Rodriguez A, Suh Y, Kim WH, Huang TT, Vijg J. Organ-specific increase in mutation accumulation and apoptosis rate in CuZn-superoxide dismutase-deficient mice. Cancer Res. 2005;65:11271–11275. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

