SR protein 9G8 modulates splicing of tau exon 10 via its proximal downstream intron, a clustering region for frontotemporal dementia mutations
- PMID: 17137791
- PMCID: PMC1866282
- DOI: 10.1016/j.mcn.2006.10.004
SR protein 9G8 modulates splicing of tau exon 10 via its proximal downstream intron, a clustering region for frontotemporal dementia mutations
Abstract
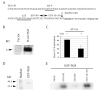
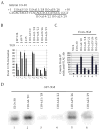
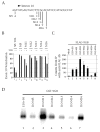
The microtubule-associated protein tau is important to normal neuronal function in the mammalian nervous system. Aggregated tau is the major component of neurofibrillary tangles (NFTs), present in several neurodegenerative diseases, including Alzheimer's and frontotemporal dementia with Parkinsonism (FTDP). Splicing misregulation of adult-specific exon 10 results in expression of abnormal ratios of tau isoforms, leading to FTDP. Positions +3 to +16 of the intron downstream of exon 10 define a clustering region for point mutations that are found in FTDP. The serine/arginine-rich (SR) factor 9G8 strongly inhibits inclusion of tau exon 10. In this study, we established that 9G8 binds directly to this clustering region, requires a wild-type residue at position +14 to inhibit exon inclusion, and RNAi constructs against 9G8 increase exon 10 inclusion. These results indicate that 9G8 plays a key role in regulation of exon 10 splicing and imply a pathogenic role in neurodegenerative diseases.
Figures



Similar articles
-
An SRp75/hnRNPG complex interacting with hnRNPE2 regulates the 5' splice site of tau exon 10, whose misregulation causes frontotemporal dementia.Gene. 2011 Oct 10;485(2):130-8. doi: 10.1016/j.gene.2011.06.020. Epub 2011 Jun 30. Gene. 2011. PMID: 21723381 Free PMC article.
-
Heterogeneous nuclear ribonucleoprotein E3 modestly activates splicing of tau exon 10 via its proximal downstream intron, a hotspot for frontotemporal dementia mutations.Gene. 2010 Feb 1;451(1-2):23-31. doi: 10.1016/j.gene.2009.11.006. Epub 2009 Nov 12. Gene. 2010. PMID: 19914360 Free PMC article.
-
A minimal length between tau exon 10 and 11 is required for correct splicing of exon 10.J Neurochem. 2004 Jul;90(1):164-72. doi: 10.1111/j.1471-4159.2004.02477.x. J Neurochem. 2004. PMID: 15198676
-
Tau gene mutations in frontotemporal dementia and parkinsonism linked to chromosome 17 (FTDP-17).Neurogenetics. 2000 Mar;2(4):193-205. doi: 10.1007/pl00022972. Neurogenetics. 2000. PMID: 10983715 Review.
-
Regulation of alternative splicing of tau exon 10.Neurosci Bull. 2014 Apr;30(2):367-77. doi: 10.1007/s12264-013-1411-2. Epub 2014 Mar 14. Neurosci Bull. 2014. PMID: 24627328 Free PMC article. Review.
Cited by
-
Cyclic AMP-dependent protein kinase regulates the alternative splicing of tau exon 10: a mechanism involved in tau pathology of Alzheimer disease.J Biol Chem. 2011 Apr 22;286(16):14639-48. doi: 10.1074/jbc.M110.204453. Epub 2011 Mar 2. J Biol Chem. 2011. PMID: 21367856 Free PMC article.
-
Alternative splicing in neurodegenerative disease and the promise of RNA therapies.Nat Rev Neurosci. 2023 Aug;24(8):457-473. doi: 10.1038/s41583-023-00717-6. Epub 2023 Jun 19. Nat Rev Neurosci. 2023. PMID: 37336982 Review.
-
Quantitative prediction of variant effects on alternative splicing in MAPT using endogenous pre-messenger RNA structure probing.Elife. 2022 Jun 13;11:e73888. doi: 10.7554/eLife.73888. Elife. 2022. PMID: 35695373 Free PMC article.
-
Functional Analysis of Mutations in Exon 9 of NF1 Reveals the Presence of Several Elements Regulating Splicing.PLoS One. 2015 Oct 28;10(10):e0141735. doi: 10.1371/journal.pone.0141735. eCollection 2015. PLoS One. 2015. PMID: 26509978 Free PMC article.
-
Single neuron transcriptomics identify SRSF/SR protein B52 as a regulator of axon growth and Choline acetyltransferase splicing.Sci Rep. 2016 Oct 11;6:34952. doi: 10.1038/srep34952. Sci Rep. 2016. PMID: 27725692 Free PMC article.
References
-
- Andreadis A. Tau gene alternative splicing: expression patterns, regulation and modulation of function in normal brain and neurodegenerative diseases. Biochem Biophys Acta. 2005;1739:91–103. - PubMed
-
- Brandt R, Hundelt M, Shahani N. Tau alteration and neuronal degeneration in tauopathies: mechanisms and models. Biochem Biophys Acta. 2005;1739:331–354. - PubMed
-
- Broderick JA, Wang J, Andreadis A. Heterogeneous nuclear ribonucleoprotein E2 binds to tau exon 10 and moderately activates its splicing. Gene. 2004;331:107–114. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

