Window Ca2+ current and its modulation by Ca2+ release in hypertrophied cardiac myocytes from dogs with chronic atrioventricular block
- PMID: 17138604
- PMCID: PMC2075376
- DOI: 10.1113/jphysiol.2006.124222
Window Ca2+ current and its modulation by Ca2+ release in hypertrophied cardiac myocytes from dogs with chronic atrioventricular block
Abstract
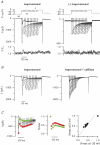
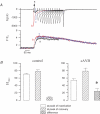
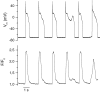
Torsades de pointes (TdP) ventricular tachycardia typically occurs in the setting of early afterdepolarizations; it contributes to arrhythmias and sudden death in congenital and acquired heart disease. Window L-type Ca2+ current (ICaL) has a central role in the arrhythmogenesis and may be particularly important under beta-adrenergic stimulation. We studied the properties of ICaL in myocytes from the dog with chronic atrioventricular block (cAVB) that has cardiac hypertrophy and an increased susceptibility to TdP. Peak ICaL densities at baseline (K+ - and Na+ -free solutions, 10 mmol l(-1) [EGTA]pip) in cAVB were comparable to control, but inactivation was shifted to the right, resulting in a larger window current area in cAVB. Under beta-adrenergic stimulation, the window current area was increased and shifted to the left, but less so in cAVB (maximum at -27 mV, versus -32 mV in control). ICaL during a step to -35 mV showed a transient reduction immediately after the peak. Test steps to 0 mV, simultaneous recording of [Ca2+]i and manipulation of sarcoplasmic reticulum (SR) Ca2+ release showed that this resulted from inhibition and fast recovery of ICaL with SR Ca2+ release. The extent of this dynamic modulation was larger in cAVB than in control (23 +/- 2% of the initially available current, versus 13 +/- 3%; P<0.05). Early afterdepolarizations (EADs) in cAVB myocytes under beta-adrenergic stimulation typically occurred in the window current voltage range and after decline of [Ca2+]i. In conclusion, in cAVB, the larger window current, its rightward shift and enhanced dynamic modulation by SR Ca2+ release may contribute to an increased incidence of EADs in cAVB under beta-adrenergic stimulation.
Figures

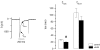




References
-
- Antzelevitch C, Shimizu W. Cellular mechanisms underlying the long QT syndrome. Curr Opin Cardiol. 2002;17:43–51. - PubMed
-
- Balke CW, Shorofsky S. Alterations in calcium handling in cardiac hypertrophy and heart failure. Cardiovasc Res. 1998;37:290–299. - PubMed
-
- Boutjdir M, Restivo M, Wei Y, Stergiopoulos K, el Sherif N. Early afterdepolarization formation in cardiac myocytes: analysis of phase plane patterns, action potential, and membrane currents. J Cardiovasc Electrophysiol. 1994;5:609–620. - PubMed
-
- Chen X, Piacentino V, Furukawa S, Goldman B, Margulies KB, Houser SR. L-type Ca2+ channel density and regulation are altered in failing human ventricular myocytes and recover after support with mechanical assist devices. Circ Res. 2002;91:517–524. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

