GABA and Gi/o differentially control circadian rhythms and synchrony in clock neurons
- PMID: 17138670
- PMCID: PMC1748197
- DOI: 10.1073/pnas.0607466103
GABA and Gi/o differentially control circadian rhythms and synchrony in clock neurons
Abstract
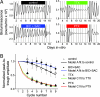
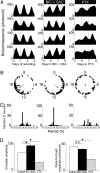
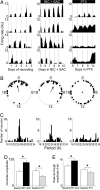
Neurons in the mammalian suprachiasmatic nuclei (SCN) generate daily rhythms in physiology and behavior, but it is unclear how they maintain and synchronize these rhythms in vivo. We hypothesized that parallel signaling pathways in the SCN are required to synchronize rhythms in these neurons for coherent output. We recorded firing and clock-gene expression patterns while blocking candidate signaling pathways for at least 8 days. GABA(A) and GABA(B) antagonism increased circadian peak firing rates and rhythm precision of cultured SCN neurons, but G(i/o) did not impair synchrony or rhythmicity. In contrast, inhibiting G(i/o) with pertussis toxin abolished rhythms in most neurons and desynchronized the population, phenocopying the loss of vasoactive intestinal polypeptide (VIP). Daily VIP receptor agonist treatment restored synchrony and rhythmicity to VIP(-/-) SCN cultures during continuous GABA receptor antagonism but not during G(i/o) blockade. Pertussis toxin did not affect circadian cycling of the liver, suggesting that G(i/o) plays a specialized role in maintaining SCN rhythmicity. We conclude that endogenous GABA controls the amplitude of SCN neuronal rhythms by reducing daytime firing, whereas G(i/o) signaling suppresses nighttime firing, and it is necessary for synchrony among SCN neurons. We propose that G(i/o), not GABA activity, converges with VIP signaling to maintain and coordinate rhythms among SCN neurons.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

