Radioimaging of light chain amyloid with a fibril-reactive monoclonal antibody
- PMID: 17138745
- PMCID: PMC1866271
Radioimaging of light chain amyloid with a fibril-reactive monoclonal antibody
Abstract
Currently, there are no available means in the United States to document objectively the location and extent of amyloid deposits in patients with systemic forms of amyloidosis. To address this limitation, we have developed a novel diagnostic strategy, namely, the use of a radiolabeled fibril-reactive murine monoclonal antibody (mAb) as an amyloid-specific imaging agent. The goal of this study was to determine the pharmacokinetics, biodistribution, and ability of this reagent to target the type of amyloid that is formed from immunoglobulin light chains, that is, AL.
Methods: Subcutaneous tumors (amyloidomas) were induced in BALB/c mice by injection of human AL fibrils. The IgG1 mAb designated 11-1F4 and an isotype-matched control antibody were radioiodinated, and the pharmacokinetics and localization of these reagents were determined from blood and tissue samples. Amyloidoma-bearing animals that received (125)I- or (124)I-labeled antibodies were imaged by whole-body small-animal SPECT/CT or small-animal PET/CT technology, respectively.
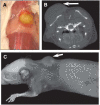
Results: Radioiodinated mAb 11-1F4 retained immunoreactivity, as evidenced by its subnanomolar affinity for light chains immobilized on 96-well microtiter plates and for beads conjugated with a light chain-related peptide. Additionally, after intravenous administration, the labeled reagents had the expected biologic half-life of murine IgG1, with monoexponential whole-body clearance kinetics. In the amyloidoma mouse model, (125)I-11-1F4 was predominately localized in the tumors, as demonstrated in biodistribution and autoradiographic analyses. The mean uptake of this reagent, that is, the percentage injected dose per gram of tissue, 72 h after injection was significantly higher for amyloid than for skeletal muscle, spleen, kidney, heart, liver, or other tissue samples. Notably, the accumulation within the amyloidomas of (125)I- or (124)I-11-1F4 was readily visible in the fused small-animal SPECT/CT or small-animal PET/CT images, respectively.
Conclusion: Our studies demonstrate the amyloid-imaging capability of a radiolabeled fibril-reactive mAb and provide the basis for a clinical trial designed to determine its diagnostic potential in patients with AL amyloidosis and other systemic amyloidoses.
Figures





References
-
- Aprile C, Marinone G, Saponaro R, Bonino C, Merlini G. Cardiac and pleuropulmonary AL amyloid imaging with technetium-99m labelled aprotinin. Eur J Nucl Med. 1995;22:1393–1401. - PubMed
-
- Hachulla E, Maulin L, Deveaux M, et al. Prospective and serial study of primary amyloidosis with serum amyloid P component scintigraphy: from diagnosis to prognosis. Am J Med. 1996;101:77–87. - PubMed
-
- Hawkins PN, Lavender JP, Pepys MB. Evaluation of systemic amyloidosis by scintigraphy with 123I-labeled serum amyloid P component. N Engl J Med. 1990;323:508–513. - PubMed
-
- Schaadt BK, Hendel HW, Gimsing P, Jonsson V, Pedersen H, Hesse B. 99mTc-Aprotinin scintigraphy in amyloidosis. J Nucl Med. 2003;44:177–183. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
