Results of a randomized international study of high-risk central nervous system B non-Hodgkin lymphoma and B acute lymphoblastic leukemia in children and adolescents
- PMID: 17138821
- PMCID: PMC1852225
- DOI: 10.1182/blood-2006-07-036665
Results of a randomized international study of high-risk central nervous system B non-Hodgkin lymphoma and B acute lymphoblastic leukemia in children and adolescents
Abstract
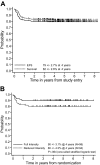
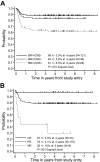
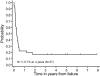
The prognosis for higher risk childhood B-cell non-Hodgkin lymphoma has improved over the past 20 years but the optimal intensity of treatment has yet to be determined. Children 21 years old or younger with newly diagnosed B-cell non-Hodgkin lymphoma/B-cell acute lymphoblastic leukemia (B-NHL/B-ALL) with higher risk factors (bone marrow [BM] with or without CNS involvement) were randomized to standard intensity French-American-British/Lymphoma Malignancy B (FAB/LMB) therapy or reduced intensity (reduced cytarabine plus etoposide and deletion of 3 maintenance courses M2, M3, M4). All patients with CNS disease had additional high-dose methotrexate (8 g/m2) plus extra intrathecal therapy. Fifty-one percent had BM involvement, 20% had CNS involvement, and 29% had BM and CNS involvement. One hundred ninety patients were randomized. The probabilities of 4-year event-free survival (EFS) and survival (S) were 79% +/- 2.7% and 82% +/- 2.6%, respectively. In patients in remission after 3 cycles who were randomized to standard versus reduced-intensity therapy, the 4-year EFS after randomization was 90% +/- 3.1% versus 80% +/- 4.2% (one-sided P = .064) and S was 93% +/- 2.7% versus 83% +/- 4.0% (one-sided P = .032). Patients with either combined BM/CNS disease at diagnosis or poor response to cyclophosphamide, Oncovin [vincristine], prednisone (COP) reduction therapy had a significantly inferior EFS and S (P < .001). Standard-intensity FAB/LMB therapy is recommended for children with high-risk B-NHL (B-ALL with or without CNS involvement).
Figures
References
-
- Cairo MS, Raetz E, Perkins SL. Non-Hodgkin lymphoma in children. In: Kufe DW, Pollock RE, Weichselbaum RR, et al., editors. Cancer Medicine. Ed. 6. London, United Kingdom: BC Decker; 2003. pp. 2337–2348.
-
- Cairo MS, Sposto R, Hoover-Regan ML, et al. Childhood and adolescent large cell lymphoma (LCL). A review of the Children's Cancer Group experience. Am J Hematol. 2003;72:53–63. - PubMed
-
- Cairo MS, Sposto R, Perkins SL, et al. Burkitt's and Burkitt-like lymphoma in children and adolescents: A review of the Children's Cancer Group Experience. Br J Haematol. 2003;120:1–11. - PubMed
-
- Patte C, Auperin A, Michon J, et al. The Societe Francaise d'Oncologie Pediatrique LMB89 protocol: highly effective multiagent chemotherapy tailored to the tumor burden and initial response in 561 unselected children with B-cell lymphomas and L3 leukemia. Blood. 2001;97:3370–3379. - PubMed
-
- Reiter A, Schrappe M, Tiemann M, et al. Improved treatment results in childhood B-cell neoplasms with tailored intensification of therapy: A report of the Berlin-Frankfurt-Munster Group Trial NHL-BFM 90. Blood. 1999;94:3294–3306. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

