Nitroxyl improves cellular heart function by directly enhancing cardiac sarcoplasmic reticulum Ca2+ cycling
- PMID: 17138943
- PMCID: PMC2769513
- DOI: 10.1161/01.RES.0000253904.53601.c9
Nitroxyl improves cellular heart function by directly enhancing cardiac sarcoplasmic reticulum Ca2+ cycling
Abstract
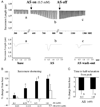
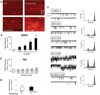
Heart failure remains a leading cause of morbidity and mortality worldwide. Although depressed pump function is common, development of effective therapies to stimulate contraction has proven difficult. This is thought to be attributable to their frequent reliance on cAMP stimulation to increase activator Ca(2+). A potential alternative is nitroxyl (HNO), the 1-electron reduction product of nitric oxide (NO) that improves contraction and relaxation in normal and failing hearts in vivo. The mechanism for myocyte effects remains unknown. Here, we show that this activity results from a direct interaction of HNO with the sarcoplasmic reticulum Ca(2+) pump and the ryanodine receptor 2, leading to increased Ca(2+) uptake and release from the sarcoplasmic reticulum. HNO increases the open probability of isolated ryanodine-sensitive Ca(2+)-release channels and accelerates Ca(2+) reuptake into isolated sarcoplasmic reticulum by stimulating ATP-dependent Ca(2+) transport. Contraction improves with no net rise in diastolic calcium. These changes are not induced by NO, are fully reversible by addition of reducing agents (redox sensitive), and independent of both cAMP/protein kinase A and cGMP/protein kinase G signaling. Rather, the data support HNO/thiolate interactions that enhance the activity of intracellular Ca(2+) cycling proteins. These findings suggest HNO donors are attractive candidates for the pharmacological treatment of heart failure.
Figures




References
-
- Houser SR, Margulies KB. Is depressed myocyte contractility centrally involved in heart failure? Circ Res. 2003;92:350–358. - PubMed
-
- Mann DL, Bristow MR. Mechanisms and models in heart failure: the biomechanical model and beyond. Circulation. 2005;111:2837–2849. - PubMed
-
- Fukuto JM, Bartberger MD, Dutton AS, Paolocci N, Wink DA, Houk KN. The physiological chemistry and biological activity of nitroxyl (HNO): the neglected, misunderstood, and enigmatic nitrogen oxide. Chem Res Toxicol. 2005;18:790–801. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- HL075265/HL/NHLBI NIH HHS/United States
- P01 HL059408/HL/NHLBI NIH HHS/United States
- P01 HL077180/HL/NHLBI NIH HHS/United States
- R01 HL047511/HL/NHLBI NIH HHS/United States
- P01HL077180/HL/NHLBI NIH HHS/United States
- R01 HL055438/HL/NHLBI NIH HHS/United States
- R37 HL030077/HL/NHLBI NIH HHS/United States
- HL47511/HL/NHLBI NIH HHS/United States
- R01 HL075265/HL/NHLBI NIH HHS/United States
- R01 HL030077/HL/NHLBI NIH HHS/United States
- P01HL59408/HL/NHLBI NIH HHS/United States
- HL30077/HL/NHLBI NIH HHS/United States
- GGP05113/TI_/Telethon/Italy
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

