GLUT4 is internalized by a cholesterol-dependent nystatin-sensitive mechanism inhibited by insulin
- PMID: 17139247
- PMCID: PMC1698906
- DOI: 10.1038/sj.emboj.7601462
GLUT4 is internalized by a cholesterol-dependent nystatin-sensitive mechanism inhibited by insulin
Abstract
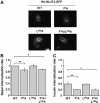
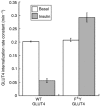
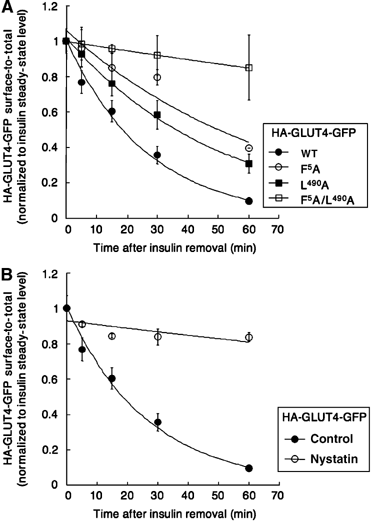
Insulin slows GLUT4 internalization by an unknown mechanism. Here we show that in unstimulated adipocytes, GLUT4 is internalized by two mechanisms. Approximately 80% of GLUT4 is internalized by a mechanism that is sensitive to the cholesterol-aggregating drug nystatin, and is independent of AP-2 clathrin adaptor and two putative GLUT4 endocytic motifs. The remaining GLUT4 is internalized by an AP-2-dependent, nystatin-resistant pathway that requires the FQQI GLUT4 motif. Insulin inhibits GLUT4 uptake by the nystatin-sensitive pathway and, consequently, GLUT4 is internalized by the AP-2-dependent pathway in stimulated adipocytes. The phenylalanine-based FQQI GLUT4 motif promotes AP-2-dependent internalization less rapidly than a tyrosine-based motif, the classic form of aromatic-based motifs. Thus, both a change in the predominant endocytosis pathway and the specific use of a suboptimal internalization motif contribute to the slowing of GLUT4 internalization in insulin-stimulated adipocytes. Insulin also inhibits the uptake of cholera-toxin B, indicating that insulin broadly regulates cholesterol-dependent uptake mechanisms rather than specially targeting GLUT4. Our work thus identifies cholesterol-dependent uptake as a novel target of insulin action in adipocytes.
Figures







References
-
- Al-Hasani H, Kunamneni RK, Dawson K, Hinck CS, Muller-Wieland D, Cushman SW (2002) Roles of the N- and C-termini of GLUT4 in endocytosis. J Cell Sci 115: 131–140 - PubMed
-
- Bonifacino JS, Traub LM (2003) Signals for sorting of transmembrane proteins to endosomes and lysosomes. Annu Rev Biochem 72: 395–447 - PubMed
-
- Choudhury A, Marks DL, Proctor KM, Gould GW, Pagano RE (2006) Regulation of caveolar endocytosis by syntaxin 6-dependent delivery of membrane components to the cell surface. Nat Cell Biol 8: 317–328 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

