Antiviral agents active against influenza A viruses
- PMID: 17139286
- PMCID: PMC7097821
- DOI: 10.1038/nrd2175
Antiviral agents active against influenza A viruses
Abstract
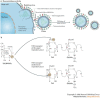
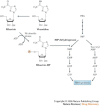
The recent outbreaks of avian influenza A (H5N1) virus, its expanding geographic distribution and its ability to transfer to humans and cause severe infection have raised serious concerns about the measures available to control an avian or human pandemic of influenza A. In anticipation of such a pandemic, several preventive and therapeutic strategies have been proposed, including the stockpiling of antiviral drugs, in particular the neuraminidase inhibitors oseltamivir (Tamiflu; Roche) and zanamivir (Relenza; GlaxoSmithKline). This article reviews agents that have been shown to have activity against influenza A viruses and discusses their therapeutic potential, and also describes emerging strategies for targeting these viruses.
Conflict of interest statement
The author declares no competing financial interests.
Figures





References
-
- Ferguson NM, et al. Strategies for containing an emerging influenza pandemic in Southeast Asia. Nature. 2005;437:209–214. - PubMed
-
- Kaye D, Pringle CR. Avian influenza viruses and their implication for human health. Clin. Infect. Dis. 2005;40:108–112. - PubMed
-
- Beigel JH, et al. Avian influenza A (H5N1) infection in humans. N. Engl. J. Med. 2005;353:1374–1385. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

