Hematopoietic stem cells
- PMID: 17141055
- PMCID: PMC2387081
- DOI: 10.1016/S0076-6879(06)19007-2
Hematopoietic stem cells
Abstract
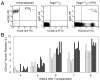
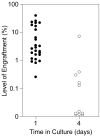
Hematopoietic stem cells (HSCs) have the capacity to self-renew and the potential to differentiate into all of the mature blood cell types. The ability to prospectively identify and isolate HSCs has been the subject of extensive investigation since the first transplantation studies implying their existence almost 50 years ago. Despite significant advances in enrichment protocols, the continuous in vitro propagation of human HSCs has not yet been achieved. This chapter describes current procedures used to phenotypically and functionally characterize candidate human HSCs and initial efforts to derive permanent human HSC lines.
Figures


Similar articles
-
Identification and in vivo analysis of murine hematopoietic stem cells.Methods Enzymol. 2010;476:429-47. doi: 10.1016/S0076-6879(10)76023-7. Methods Enzymol. 2010. PMID: 20691879
-
Ex vivo expansion of murine and human hematopoietic stem cells.Methods Mol Biol. 2014;1185:211-21. doi: 10.1007/978-1-4939-1133-2_14. Methods Mol Biol. 2014. PMID: 25062631
-
Hematopoietic stem cell enrichment from the AGM region of the mouse embryo.Methods Mol Med. 2005;105:257-72. doi: 10.1385/1-59259-826-9:257. Methods Mol Med. 2005. PMID: 15492400 Review.
-
New approaches to expand hematopoietic stem and progenitor cells.Expert Opin Biol Ther. 2012 Jun;12(6):743-56. doi: 10.1517/14712598.2012.681372. Epub 2012 Apr 23. Expert Opin Biol Ther. 2012. PMID: 22519359 Review.
-
Flk-2 is a marker in hematopoietic stem cell differentiation: a simple method to isolate long-term stem cells.Proc Natl Acad Sci U S A. 2001 Dec 4;98(25):14541-6. doi: 10.1073/pnas.261562798. Epub 2001 Nov 27. Proc Natl Acad Sci U S A. 2001. PMID: 11724967 Free PMC article.
Cited by
-
Journey of micronanoplastics with blood components.RSC Adv. 2023 Oct 27;13(45):31435-31459. doi: 10.1039/d3ra05620a. eCollection 2023 Oct 26. RSC Adv. 2023. PMID: 37901269 Free PMC article. Review.
-
ANTXR1 Regulates Erythroid Cell Proliferation and Differentiation through wnt/β-Catenin Signaling Pathway In Vitro and in Hematopoietic Stem Cell.Dis Markers. 2022 Aug 27;2022:1226697. doi: 10.1155/2022/1226697. eCollection 2022. Dis Markers. 2022. PMID: 36065334 Free PMC article.
-
Stem cell-based therapy for systemic sclerosis.Rheumatol Adv Pract. 2023 Nov 20;7(3):rkad101. doi: 10.1093/rap/rkad101. eCollection 2023. Rheumatol Adv Pract. 2023. PMID: 38075180 Free PMC article. Review.
-
Gene and cell therapy for heart failure.Antioxid Redox Signal. 2009 Aug;11(8):2025-42. doi: 10.1089/ars.2009.2495. Antioxid Redox Signal. 2009. PMID: 19416058 Free PMC article. Review.
-
Bioluminescence Imaging and ICP-MS Associated with SPION as a Tool for Hematopoietic Stem and Progenitor Cells Homing and Engraftment Evaluation.Pharmaceutics. 2023 Mar 3;15(3):828. doi: 10.3390/pharmaceutics15030828. Pharmaceutics. 2023. PMID: 36986690 Free PMC article.
References
-
- Alexander WS, Roberts AW, Nicola NA, Li R, Metcalf D. Deficiencies in progenitor cells of multiple hematopoietic lineages and defective megakaryocytopoiesis in mice lacking the thrombopoietic receptor c-Mpl. Blood. 1996;87:2162–2170. - PubMed
-
- Ando K, Yahata T, Sato T, Miyatake H, Matsuzawa H, Oki M, Miyoshi H, Tsuji T, Kato S, Hotta T. Direct evidence for ex vivo expansion of human hematopoietic stem cells. Blood. 2006;107:3371–3377. - PubMed
-
- Angelopoulou MK, Rinder H, Wang C, Burtness B, Cooper DL, Krause DS. A preclinical xenotransplantation animal model to assess human hematopoietic stem cell engraftment. Transfusion. 2004;44:555–566. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

