Sef and Sprouty expression in the developing ocular lens: implications for regulating lens cell proliferation and differentiation
- PMID: 17141539
- PMCID: PMC1847394
- DOI: 10.1016/j.semcdb.2006.10.007
Sef and Sprouty expression in the developing ocular lens: implications for regulating lens cell proliferation and differentiation
Abstract
In many developmental systems, growth factor signalling must be temporally and spatially regulated, and this is commonly achieved by growth factor antagonists. Here, we describe the expression patterns of newly identified growth factor inhibitors, Sprouty and Sef, in the developing ocular lens. Sprouty and Sef are both expressed in the lens throughout embryogenesis, and become restricted to the lens epithelium, indicating that lens cell proliferation and fibre differentiation may be tightly regulated by such antagonists. Future studies will be aimed at understanding how these negative regulatory molecules modulate growth factor-induced signalling pathways and cellular processes in the lens.
Figures

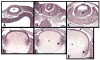


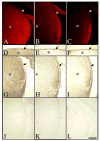
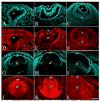

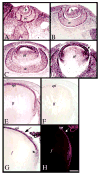

References
-
- McAvoy JW, Chamberlain C, de Iongh R, Hales A, Lovicu FJ. Lens Development. Eye. 1999;13:425–37. - PubMed
-
- Robinson ML, Lovicu FJ. The Lens: Historical and Comparative Perspectives. In: Lovicu FJ, Robinson ML, editors. Development of the Ocular Lens. New York: Cambridge University Press; 2004. pp. 3–26.
-
- Lovicu FJ, McAvoy JW. Growth Factor Regulation Of Lens Development. Dev Biol. 2005;280:1–14. - PubMed
-
- Schulz MW, Chamberlain CG, de Iongh RU, McAvoy JW. Acidic and basic FGF in ocular media and lens: implications for lens polarity and growth patterns. Development. 1993;118:117–26. - PubMed
-
- McAvoy JW, Chamberlain CG. Fibroblast growth factor (FGF) induces different responses in lens epithelial cells depending on its concentration. Development. 1989;107:221–28. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

