On the decrease in lateral mobility of phospholipids by sugars
- PMID: 17142271
- PMCID: PMC1796821
- DOI: 10.1529/biophysj.106.096461
On the decrease in lateral mobility of phospholipids by sugars
Abstract
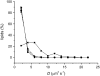
Upon cold and drought stress, sucrose and trehalose protect membrane structures from fusion and leakage. Similarly, these sugars protect membrane proteins from inactivation during dehydration. We studied the interactions between sugars and phospholipid membranes in giant unilamellar vesicles with the fluorescent lipid analog 3,3'-dioctadecyloxacarbocyanine perchlorate incorporated. Using fluorescence correlation spectroscopy, it was found that sucrose decreased the lateral mobility of phospholipids in the fully rehydrated, liquid crystalline membrane more than other sugars did, including trehalose. To describe the nature of the difference in the interaction of phospholipids with sucrose and trehalose, atomistic molecular dynamics studies were performed. Simulations up to 100 ns showed that sucrose interacted with more phospholipid headgroups simultaneously than trehalose, resulting in a larger decrease of the lateral mobility. Using coarse-grained molecular dynamics, we show that this increase in interactions can lead to a relatively large decrease in lateral phospholipid mobility.
Figures


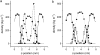


References
-
- Crowe, J. H., J. F. Carpenter, and L. M. Crowe. 1997. The role of vitrification in anhydrobiosis. Annu. Rev. Physiol. 60:73–103. - PubMed
-
- Hounsa, C. G., E. V. Brandt, J. Thevelein, S. Hohmann, and B. A. Prior. 1998. Role of trehalose in survival of Saccharomyces cerevisiae under osmotic stress. Microbiology. 144:671–680. - PubMed
-
- Wonisch, W., M. Hayn, R. J. Schaur, F. Tatzber, I. Kranner, D. Grill, R. Winkler, T. Bilinski, S. D. Kohlwein, and H. Esterbauer. 1997. Increased stress parameter synthesis in the yeast Saccharomyces cerevisiae after treatment with 4-hydroxy-2-nonenal. FEBS Lett. 405:11–15. - PubMed
-
- Oliver, A. E., D. K. Hincha, and J. H. Crowe. 2002. Looking beyond sugars: the role of amphiphilic solutes in preventing adventitious reactions in anhydrobiotes at low water contents. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 131:515–525. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

