Structure of the first transmembrane domain of the neuronal acetylcholine receptor beta2 subunit
- PMID: 17142275
- PMCID: PMC1796834
- DOI: 10.1529/biophysj.106.095364
Structure of the first transmembrane domain of the neuronal acetylcholine receptor beta2 subunit
Abstract
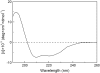
The recent cryoelectron microscopy structure of the Torpedo nicotinic acetylcholine receptor (nAChR) at 4-A resolution shows long helices for all transmembrane (TM) domains. This is in disagreement with several previous reports that the first TM domain of nAChR and other Cys-loop receptors are not entirely helical. In this study, we determined the structure and backbone dynamics of an extended segment encompassing the first TM domain (TM1e) of nAChR beta(2) subunit in dodecylphosphocholine micelles, using solution-state NMR and circular dichroism (CD) spectroscopy. Both CD and NMR results show less helicity in TM1e than in Torpedo nAChR structure (Protein Data Bank: 2BG9). The helical ending residues at the C-terminus are the same in the TM1e NMR structure and the Torpedo nAChR structure, but the helical starting residue (I-217) in TM1e is seven residues closer to the C-terminus. Interestingly, the helical starting residue is two residues before the highly conserved P-219, in accordance with the hypothesis that proline causes helical distortions at three residues preceding it. The NMR relaxation measurements show a dynamics pattern consistent with TM1e structure. The substantial nonhelical content adds greater flexibilities to TM1e, thereby implicating a different molecular basis for nAChR function compared to a longer and more rigid helical TM1.
Figures




References
-
- Karlin, A. 2002. Emerging structure of the nicotinic acetylcholine receptors. Nat. Rev. Neurosci. 3:102–114. - PubMed
-
- Lester, H. A., M. I. Dibas, D. S. Dahan, J. F. Leite, and D. A. Dougherty. 2004. Cys-loop receptors: new twists and turns. Trends Neurosci. 27:329–336. - PubMed
-
- Sine, S. M., and A. G. Engel. 2006. Recent advances in Cys-loop receptor structure and function. Nature. 440:448–455. - PubMed
-
- Brejc, K., W. J. van Dijk, R. V. Klaassen, M. Schuurmans, J. van Der Oost, A. B. Smit, and T. K. Sixma. 2001. Crystal structure of an ACh-binding protein reveals the ligand-binding domain of nicotinic receptors. Nature. 411:269–276. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

