Sound sequence discrimination learning motivated by reward requires dopaminergic D2 receptor activation in the rat auditory cortex
- PMID: 17142301
- PMCID: PMC1783622
- DOI: 10.1101/lm.390506
Sound sequence discrimination learning motivated by reward requires dopaminergic D2 receptor activation in the rat auditory cortex
Abstract
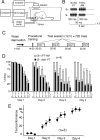
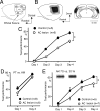
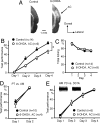
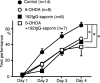
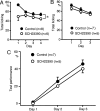
We have previously reported that sound sequence discrimination learning requires cholinergic inputs to the auditory cortex (AC) in rats. In that study, reward was used for motivating discrimination behavior in rats. Therefore, dopaminergic inputs mediating reward signals may have an important role in the learning. We tested the possibility in the present study. Rats were trained to discriminate sequences of two sound components, and licking behavior in response to one of the two sequences was rewarded with water. To identify the dopaminergic inputs responsible for the learning, dopaminergic afferents to the AC were lesioned with local injection of 6-hydroxydopamine (6-OHDA). The injection attenuated sound sequence discrimination learning, while it had no effect on discrimination between the sound components of the sequence stimuli. Local injection of 6-OHDA into the nucleus accumbens attenuated sound discrimination learning. However, not only discrimination learning of sound sequence but also that of the sound components were impaired. SCH23390 (0.2 mg/kg, i.p.), a D1 receptor antagonist, had no effect on sound sequence discrimination learning, while it attenuated the licking behavior to unfamiliar stimuli. Haloperidol (0.5 mg/kg, i.p.), a D2 family antagonist, attenuated sound sequence discrimination learning, while it had no clear suppressive effect on discrimination of two different sound components and licking. These results suggest that D2 family receptors activated by dopaminergic inputs to the AC are required for sound sequence discrimination learning.
Figures




Similar articles
-
Sound sequence discrimination learning is dependent on cholinergic inputs to the rat auditory cortex.Neurosci Res. 2004 Sep;50(1):113-23. doi: 10.1016/j.neures.2004.06.007. Neurosci Res. 2004. PMID: 15288504
-
Auditory discrimination training rescues developmentally degraded directional selectivity and restores mature expression of GABA(A) and AMPA receptor subunits in rat auditory cortex.Behav Brain Res. 2012 Apr 15;229(2):301-7. doi: 10.1016/j.bbr.2011.12.041. Epub 2012 Jan 26. Behav Brain Res. 2012. PMID: 22306199
-
Characteristics of sound discrimination enhancement after sound exposure in adult rats.Behav Neurosci. 2005 Aug;119(4):961-73. doi: 10.1037/0735-7044.119.4.961. Behav Neurosci. 2005. PMID: 16187825
-
Behavioral semantics of learning and crossmodal processing in auditory cortex: the semantic processor concept.Hear Res. 2011 Jan;271(1-2):3-15. doi: 10.1016/j.heares.2010.10.006. Epub 2010 Oct 29. Hear Res. 2011. PMID: 20971178 Review.
-
Learning induces unique transcriptional landscapes in the auditory cortex.Hear Res. 2023 Oct;438:108878. doi: 10.1016/j.heares.2023.108878. Epub 2023 Aug 26. Hear Res. 2023. PMID: 37659220 Free PMC article. Review.
Cited by
-
Neural and behavioral investigations into timbre perception.Front Syst Neurosci. 2013 Nov 13;7:88. doi: 10.3389/fnsys.2013.00088. Front Syst Neurosci. 2013. PMID: 24312021 Free PMC article. Review.
-
Set and setting: how behavioral state regulates sensory function and plasticity.Neurobiol Learn Mem. 2013 Nov;106:1-10. doi: 10.1016/j.nlm.2013.06.007. Epub 2013 Jun 19. Neurobiol Learn Mem. 2013. PMID: 23792020 Free PMC article. Review.
-
Models of Acetylcholine and Dopamine Signals Differentially Improve Neural Representations.Front Comput Neurosci. 2017 Jun 22;11:54. doi: 10.3389/fncom.2017.00054. eCollection 2017. Front Comput Neurosci. 2017. PMID: 28690509 Free PMC article.
-
Dissecting natural sensory plasticity: hormones and experience in a maternal context.Hear Res. 2009 Jun;252(1-2):21-8. doi: 10.1016/j.heares.2009.04.014. Epub 2009 May 3. Hear Res. 2009. PMID: 19401225 Free PMC article. Review.
-
Dopaminergic modulation of auditory cortex-dependent memory consolidation through mTOR.Cereb Cortex. 2008 Nov;18(11):2646-58. doi: 10.1093/cercor/bhn026. Epub 2008 Mar 4. Cereb Cortex. 2008. PMID: 18321872 Free PMC article.
References
-
- Amalric M., Koob G.F. Functionally selective neurochemical afferents and efferents of the mesocorticolimbic and nigrostriatal dopamine system. Prog. Brain Res. 1993;99:209–226. - PubMed
-
- Bao S., Chan V.T., Merzenich M.M. Cortical remodelling induced by activity of ventral tegmental dopamine neurons. Nature. 2001;412:79–83. - PubMed
-
- Barnett A., Iorio L.C., McQuade R., Chipkin R.E. Pharmacological and behavioral effects of D1 dopamine antagonists. Adv. Exp. Med. Biol. 1988;235:137–144. - PubMed
-
- Blond O., Crepel F., Otani S. Long-term potentiation in rat prefrontal slices facilitated by phased application of dopamine. Eur. J. Pharmacol. 2002;438:115–116. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
