Associative memory in three aplysiids: correlation with heterosynaptic modulation
- PMID: 17142308
- PMCID: PMC1783637
- DOI: 10.1101/lm.284006
Associative memory in three aplysiids: correlation with heterosynaptic modulation
Abstract
Much recent research on mechanisms of learning and memory focuses on the role of heterosynaptic neuromodulatory signaling. Such neuromodulation appears to stabilize Hebbian synaptic changes underlying associative learning, thereby extending memory. Previous comparisons of three related sea-hares (Mollusca, Opisthobranchia) uncovered interspecific variation in neuromodulatory signaling: strong in Aplysia californica, immeasureable in Dolabrifera dolabrifera, and intermediate in Phyllaplysia taylori. The present study addressed whether this interspecific variation in neuromodulation is correlated with memory of associative (classical conditioning) learning. We differentially conditioned the tail-mantle withdrawal reflex of each of the three species: Mild touch to one side of the tail was paired with a noxious electrical stimulus to the neck. Mild touch to the other side served as an internal control. Post-training reflex amplitudes were tested 15-30 min after training and compared with pre-test amplitudes. All three species showed conditioning: training increased the paired reflex more than the unpaired reflex. However, the temporal pattern of conditioning varied between species. Aplysia showed modest conditioning that grew across the post-test period. Dolabrifera showed distinctly short-lived conditioning, present only on the first post-test. The time course of memory in Phyllaplysia was intermediate, although not statistically distinguishable from the other two species. Taken together, these experiments suggest that evolutionary changes in nonassociative heterosynaptic modulation may contribute to evolutionary changes in the stability of the memory of classical conditioning.
Figures
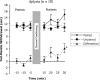
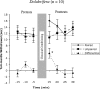
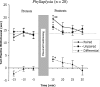

Similar articles
-
Dissociation between sensitization and learning-related neuromodulation in an aplysiid species.J Comp Neurol. 1999 Jun 14;408(4):506-14. doi: 10.1002/(sici)1096-9861(19990614)408:4<506::aid-cne5>3.0.co;2-p. J Comp Neurol. 1999. PMID: 10340501
-
Evolution of learning in three aplysiid species: differences in heterosynaptic plasticity contrast with conservation in serotonergic pathways.J Physiol. 2003 Jul 1;550(Pt 1):241-53. doi: 10.1113/jphysiol.2003.038356. Epub 2003 May 9. J Physiol. 2003. PMID: 12740422 Free PMC article.
-
Evolution of nonassociative learning: behavioral analysis of a phylogenetic lesion.Neurobiol Learn Mem. 1998 May;69(3):326-37. doi: 10.1006/nlme.1998.3829. Neurobiol Learn Mem. 1998. PMID: 9707494
-
Is heterosynaptic modulation essential for stabilizing Hebbian plasticity and memory?Nat Rev Neurosci. 2000 Oct;1(1):11-20. doi: 10.1038/35036191. Nat Rev Neurosci. 2000. PMID: 11252764 Review.
-
Molecular mechanisms of associative learning in mammal and mollusc.J Physiol (Paris). 1988-1989;83(3):119-25. J Physiol (Paris). 1988. PMID: 2483171 Review.
Cited by
-
Biodiversity Meets Neuroscience: From the Sequencing Ship (Ship-Seq) to Deciphering Parallel Evolution of Neural Systems in Omic's Era.Integr Comp Biol. 2015 Dec;55(6):1005-17. doi: 10.1093/icb/icv084. Epub 2015 Jul 10. Integr Comp Biol. 2015. PMID: 26163680 Free PMC article. Review.
-
NSF workshop report: discovering general principles of nervous system organization by comparing brain maps across species.Brain Behav Evol. 2014;83(1):1-8. doi: 10.1159/000360152. Epub 2014 Feb 28. Brain Behav Evol. 2014. PMID: 24603302 Free PMC article.
-
The Transition to Minimal Consciousness through the Evolution of Associative Learning.Front Psychol. 2016 Dec 22;7:1954. doi: 10.3389/fpsyg.2016.01954. eCollection 2016. Front Psychol. 2016. PMID: 28066282 Free PMC article.
-
Parallel evolution of serotonergic neuromodulation underlies independent evolution of rhythmic motor behavior.J Neurosci. 2013 Feb 6;33(6):2709-17. doi: 10.1523/JNEUROSCI.4196-12.2013. J Neurosci. 2013. PMID: 23392697 Free PMC article.
References
-
- Almaguer-Meliana W., Rojas-Reyesa Y., Alvarea A., Rosilloa J.C., Frey J.U., Bergadoa J.A. Long-term potentiation in the dentate gyrus in freely moving rats is reinforced by intraventricular application of norepinephrine, but not oxotremorine. Neurobiol. Learn. Mem. 2005;83:72–78. - PubMed
-
- Bailey C.H., Giustetto M., Huang Y.-Y., Hawkins R.D., Kandel E.R. Is heterosynaptic modulation essential for stabilizing hebbian plasticity and memory? Nat. Rev. Neurosci. 2000a;1:11–20. - PubMed
-
- Bailey C.H., Giustetto M., Zhu H., Chen M., Kandel E.R. A novel function for serotonin-mediated short-term facilitation in Aplysia: Conversion of a transient cell-wide homosynaptic Hebbian plasticity into a persistent protein synthesis independent synapse-specific enhancement. Proc. Natl. Acad. Sci. 2000b;97:11581–11586. - PMC - PubMed
-
- Bristol A.S., Sutton M.A., Carew T.J. Neural circuit of tail-elicited siphon withdrawal in Aplysia. I. Differential lateralization of sensitization and dishabituation. J. Neurophysiol. 2004;91:666–677. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
