ESET/SETDB1 gene expression and histone H3 (K9) trimethylation in Huntington's disease
- PMID: 17142323
- PMCID: PMC1748195
- DOI: 10.1073/pnas.0606373103
ESET/SETDB1 gene expression and histone H3 (K9) trimethylation in Huntington's disease
Abstract
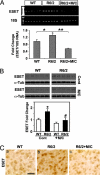
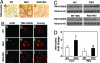
Chromatin remodeling and transcription regulation are tightly controlled under physiological conditions. It has been suggested that altered chromatin modulation and transcription dysfunction may play a role in the pathogenesis of Huntington's disease (HD). Increased histone methylation, a well established mechanism of gene silencing, results in transcriptional repression. ERG-associated protein with SET domain (ESET), a histone H3 (K9) methyltransferase, mediates histone methylation. We show that ESET expression is markedly increased in HD patients and in transgenic R6/2 HD mice. Similarly, the protein level of trimethylated histone H3 (K9) was also elevated in HD patients and in R6/2 mice. We further demonstrate that both specificity protein 1 (Sp1) and specificity protein 3 (Sp3) act as transcriptional activators of the ESET promoter in neurons and that mithramycin, a clinically approved guanosine-cytosine-rich DNA binding antitumor antibiotic, interferes with the DNA binding of these Sp family transcription factors, suppressing basal ESET promoter activity in a dose dependent manner. The combined pharmacological treatment with mithramycin and cystamine down-regulates ESET gene expression and reduces hypertrimethylation of histone H3 (K9). This polytherapy significantly ameliorated the behavioral and neuropathological phenotype in the R6/2 mice and extended survival over 40%, well beyond any existing reported treatment in HD mice. Our data suggest that modulation of gene silencing mechanisms, through regulation of the ESET gene is important to neuronal survival and, as such, may be a promising treatment in HD patients.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

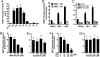

Similar articles
-
Monoallele deletion of CBP leads to pericentromeric heterochromatin condensation through ESET expression and histone H3 (K9) methylation.Hum Mol Genet. 2008 Jun 15;17(12):1774-82. doi: 10.1093/hmg/ddn067. Epub 2008 Mar 4. Hum Mol Genet. 2008. PMID: 18319327 Free PMC article.
-
mAM facilitates conversion by ESET of dimethyl to trimethyl lysine 9 of histone H3 to cause transcriptional repression.Mol Cell. 2003 Aug;12(2):475-87. doi: 10.1016/j.molcel.2003.08.007. Mol Cell. 2003. PMID: 14536086
-
Histone H3-K9 methyltransferase ESET is essential for early development.Mol Cell Biol. 2004 Mar;24(6):2478-86. doi: 10.1128/MCB.24.6.2478-2486.2004. Mol Cell Biol. 2004. PMID: 14993285 Free PMC article.
-
Epigenetics of Huntington's Disease.Adv Exp Med Biol. 2017;978:277-299. doi: 10.1007/978-3-319-53889-1_15. Adv Exp Med Biol. 2017. PMID: 28523552 Review.
-
[The roles of histone lysine methylation in epigenetic regulation].Yi Chuan. 2007 Apr;29(4):387-92. doi: 10.1360/yc-007-0387. Yi Chuan. 2007. PMID: 17548299 Review. Chinese.
Cited by
-
Mechanisms Underlying Brain Aging Under Normal and Pathological Conditions.Neurosci Bull. 2023 Feb;39(2):303-314. doi: 10.1007/s12264-022-00969-9. Epub 2022 Nov 27. Neurosci Bull. 2023. PMID: 36437436 Free PMC article. Review.
-
SETDB1 in cancer: overexpression and its therapeutic implications.Am J Cancer Res. 2021 May 15;11(5):1803-1827. eCollection 2021. Am J Cancer Res. 2021. PMID: 34094655 Free PMC article. Review.
-
ATRX induction by mutant huntingtin via Cdx2 modulates heterochromatin condensation and pathology in Huntington's disease.Cell Death Differ. 2012 Jul;19(7):1109-16. doi: 10.1038/cdd.2011.196. Epub 2012 Jan 13. Cell Death Differ. 2012. PMID: 22240898 Free PMC article.
-
Histone methylation in the nervous system: functions and dysfunctions.Mol Neurobiol. 2013 Apr;47(2):740-56. doi: 10.1007/s12035-012-8376-4. Epub 2012 Nov 17. Mol Neurobiol. 2013. PMID: 23161382 Review.
-
Non-Cell Autonomous and Epigenetic Mechanisms of Huntington's Disease.Int J Mol Sci. 2021 Nov 19;22(22):12499. doi: 10.3390/ijms222212499. Int J Mol Sci. 2021. PMID: 34830381 Free PMC article. Review.
References
-
- Cha JH. Trends Neurosci. 2000;23:387–392. - PubMed
-
- Nucifora FC, Jr, Sasaki M, Peters MF, Huang H, Cooper JK, Yamada M, Takahashi H, Tsuji S, Troncoso J, Dawson VL, et al. Science. 2001;291:2423–2428. - PubMed
-
- Dunah AW, Jeong H, Griffin A, Kim YM, Standaert DG, Hersch SM, Mouradian MM, Young AB, Tanese N, Krainc D. Science. 2002;296:2238–2243. - PubMed
-
- Steffan JS, Bodai L, Pallos J, Poelman M, McCampbell A, Apostol BL, Kazantsev A, Schmidt E, Zhu YZ, Greenwald M. Nature. 2001;413:739–743. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

