Ca2+-dependent and -independent abscisic acid activation of plasma membrane anion channels in guard cells of Nicotiana tabacum
- PMID: 17142476
- PMCID: PMC1761993
- DOI: 10.1104/pp.106.092643
Ca2+-dependent and -independent abscisic acid activation of plasma membrane anion channels in guard cells of Nicotiana tabacum
Abstract
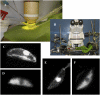
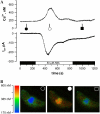
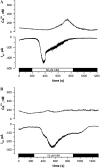
Drought induces stomatal closure, a response that is associated with the activation of plasma membrane anion channels in guard cells, by the phytohormone abscisic acid (ABA). In several species, this response is associated with changes in the cytoplasmic free Ca(2+) concentration. In Vicia faba, however, guard cell anion channels activate in a Ca(2+)-independent manner. Because of potential differences between species, Nicotiana tabacum guard cells were studied in intact plants, with simultaneous recordings of the plasma membrane conductance and the cytoplasmic free Ca(2+) concentration. ABA triggered transient rises in cytoplasmic Ca(2+) in the majority of the guard cells (14 out of 19). In seven out of 14 guard cells, the change in cytoplasmic free Ca(2+) closely matched the activation of anion channels, while the Ca(2+) rise was delayed in seven other cells. In the remaining five cells, ABA stimulated anion channels without a change in the cytoplasmic Ca(2+) level. Even though ABA could activate anion channels in N. tabacum guard cells independent of a rise in the cytoplasmic Ca(2+) concentration, patch clamp experiments showed that anion channels in these cells are stimulated by elevated Ca(2+) in an ATP-dependent manner. Guard cells thus seem to have evolved both Ca(2+)-independent and -dependent ABA signaling pathways. Guard cells of N. tabacum apparently utilize both pathways, while ABA signaling in V. faba seems to be restricted to the Ca(2+)-independent pathway.
Figures




Similar articles
-
Cytosolic abscisic acid activates guard cell anion channels without preceding Ca2+ signals.Proc Natl Acad Sci U S A. 2005 Mar 15;102(11):4203-8. doi: 10.1073/pnas.0500146102. Epub 2005 Mar 7. Proc Natl Acad Sci U S A. 2005. PMID: 15753314 Free PMC article.
-
Signal transduction and ion channels in guard cells.Philos Trans R Soc Lond B Biol Sci. 1998 Sep 29;353(1374):1475-88. doi: 10.1098/rstb.1998.0303. Philos Trans R Soc Lond B Biol Sci. 1998. PMID: 9800209 Free PMC article. Review.
-
Silencing of NtMPK4 impairs CO-induced stomatal closure, activation of anion channels and cytosolic Casignals in Nicotiana tabacum guard cells.Plant J. 2008 Aug;55(4):698-708. doi: 10.1111/j.1365-313X.2008.03542.x. Epub 2008 Apr 30. Plant J. 2008. PMID: 18452588
-
ABA depolarizes guard cells in intact plants, through a transient activation of R- and S-type anion channels.Plant J. 2004 Feb;37(4):578-88. doi: 10.1111/j.1365-313x.2003.01985.x. Plant J. 2004. PMID: 14756768
-
Guard cell sensory systems: recent insights on stomatal responses to light, abscisic acid, and CO2.Curr Opin Plant Biol. 2016 Oct;33:157-167. doi: 10.1016/j.pbi.2016.07.003. Epub 2016 Aug 9. Curr Opin Plant Biol. 2016. PMID: 27518594 Review.
Cited by
-
Abscisic acid and CO2 signalling via calcium sensitivity priming in guard cells, new CDPK mutant phenotypes and a method for improved resolution of stomatal stimulus-response analyses.Ann Bot. 2012 Jan;109(1):5-17. doi: 10.1093/aob/mcr252. Epub 2011 Oct 12. Ann Bot. 2012. PMID: 21994053 Free PMC article. Review.
-
Regulation of reactive oxygen species-mediated abscisic acid signaling in guard cells and drought tolerance by glutathione.Front Plant Sci. 2013 Nov 20;4:472. doi: 10.3389/fpls.2013.00472. eCollection 2013. Front Plant Sci. 2013. PMID: 24312112 Free PMC article.
-
Mutations in the SLAC1 anion channel slow stomatal opening and severely reduce K+ uptake channel activity via enhanced cytosolic [Ca2+] and increased Ca2+ sensitivity of K+ uptake channels.New Phytol. 2013 Jan;197(1):88-98. doi: 10.1111/nph.12008. Epub 2012 Nov 5. New Phytol. 2013. PMID: 23126621 Free PMC article.
-
Advances and prospects of rhodopsin-based optogenetics in plant research.Plant Physiol. 2021 Oct 5;187(2):572-589. doi: 10.1093/plphys/kiab338. Plant Physiol. 2021. PMID: 35237820 Free PMC article. Review.
-
Activity of guard cell anion channel SLAC1 is controlled by drought-stress signaling kinase-phosphatase pair.Proc Natl Acad Sci U S A. 2009 Dec 15;106(50):21425-30. doi: 10.1073/pnas.0912021106. Epub 2009 Dec 2. Proc Natl Acad Sci U S A. 2009. PMID: 19955405 Free PMC article.
References
-
- Allen GJ, Chu SP, Harrington CL, Schumacher K, Hoffmann T, Tang YY, Grill E, Schroeder JI (2001) A defined range of guard cell calcium oscillation parameters encodes stomatal movements. Nature 411 1053–1057 - PubMed
-
- Allen GJ, Chu SP, Schumacher K, Shimazaki CT, Vafeados D, Kemper A, Hawke SD, Tallman G, Tsien RY, Harper JF, et al (2000) Alteration of stimulus-specific guard cell calcium oscillations and stomatal closing in Arabidopsis det3 mutant. Science 289 2338–2342 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

