Essential role for Co-chaperone Fkbp52 but not Fkbp51 in androgen receptor-mediated signaling and physiology
- PMID: 17142810
- PMCID: PMC2577319
- DOI: 10.1074/jbc.M609360200
Essential role for Co-chaperone Fkbp52 but not Fkbp51 in androgen receptor-mediated signaling and physiology
Abstract
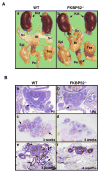
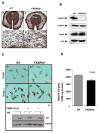
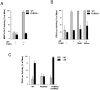
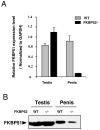
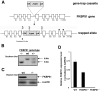
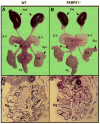
Fkbp52 and Fkbp51 are tetratricopeptide repeat proteins found in steroid receptor complexes, and Fkbp51 is an androgen receptor (AR) target gene. Although in vitro studies suggest that Fkbp52 and Fkbp51 regulate hormone binding and/or subcellular trafficking of receptors, the roles of Fkbp52 and Fkbp51 in vivo have not been extensively investigated. Here, we evaluate their physiological roles in Fkbp52-deficient and Fkbp51-deficient mice. Fkbp52-deficient males developed defects in select reproductive organs (e.g. penile hypospadias and prostate dysgenesis but normal testis), pointing to a role for Fkbp52 in AR-mediated signaling and function. Surprisingly, ablation of Fkbp52 did not affect AR hormone binding or nuclear translocation in vivo and in vitro. Molecular studies in mouse embryonic fibroblast cells uncovered that Fkbp52 is critical to AR transcriptional activity. Interestingly, Fkbp51 expression was down-regulated in Fkbp52-deficient males but only in affected tissues, providing further evidence of tissue-specific loss of AR activity and suggesting that Fkbp51 is an AR target gene essential to penile and prostate development. However, Fkbp51-deficient mice were normal, showing no defects in AR-mediated reproductive function. Our work demonstrates that Fkbp52 but not Fkbp51 is essential to AR-mediated signaling and provides evidence for an unprecedented Fkbp52 function, direct control of steroid receptor transcriptional activity.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

