Structure of HLA-A*1101 in complex with a hepatitis B peptide homologue
- PMID: 17142892
- PMCID: PMC2225367
- DOI: 10.1107/S1744309106044228
Structure of HLA-A*1101 in complex with a hepatitis B peptide homologue
Abstract
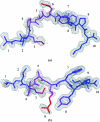
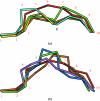
A high-resolution structure of the human MHC-I molecule HLA-A*1101 is presented in which it forms a complex with a sequence homologue of a peptide that occurs naturally in hepatitis B virus DNA polymerase. The sequence of the bound peptide is AIMPARFYPK, while that of the corresponding natural peptide is LIMPARFYPK. The peptide does not make efficient use of the middle E pocket for binding, which leads to a rather superficial and exposed binding mode for the central peptide residues. Despite this, the peptide binds with high affinity (IC50 of 31 nM).
Figures

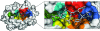
Similar articles
-
Effect of anchor residue modifications on the stability of HLA-A11/peptide complexes.Biochem Biophys Res Commun. 1995 Jan 5;206(1):8-14. doi: 10.1006/bbrc.1995.1002. Biochem Biophys Res Commun. 1995. PMID: 7818553
-
Atomic structure of a human MHC molecule presenting an influenza virus peptide.Nature. 1992 Nov 26;360(6402):367-9. doi: 10.1038/360367a0. Nature. 1992. PMID: 1448154
-
Classical and nonclassical class I major histocompatibility complex molecules exhibit subtle conformational differences that affect binding to CD8alphaalpha.J Biol Chem. 2000 May 19;275(20):15232-8. doi: 10.1074/jbc.275.20.15232. J Biol Chem. 2000. PMID: 10809759
-
Outsize peptides bulge out of the groove.Immunol Today. 1993 Feb;14(2):51-2. doi: 10.1016/0167-5699(93)90057-r. Immunol Today. 1993. PMID: 8447932 Review.
-
Gorillas with spondyloarthropathies express an MHC class I molecule with only limited sequence similarity to HLA-B27 that binds peptides with arginine at P2.J Immunol. 2001 Mar 1;166(5):3334-44. doi: 10.4049/jimmunol.166.5.3334. J Immunol. 2001. PMID: 11207289 Review.
Cited by
-
Structural and functional distinctiveness of HLA-A2 allelic variants.Immunol Res. 2012 Sep;53(1-3):182-90. doi: 10.1007/s12026-012-8295-5. Immunol Res. 2012. PMID: 22434516 Review.
-
Humidity control as a strategy for lattice optimization applied to crystals of HLA-A*1101 complexed with variant peptides from dengue virus.Acta Crystallogr Sect F Struct Biol Cryst Commun. 2007 May 1;63(Pt 5):386-92. doi: 10.1107/S1744309107013693. Epub 2007 Apr 6. Acta Crystallogr Sect F Struct Biol Cryst Commun. 2007. PMID: 17565177 Free PMC article.
-
Structure of a SARS coronavirus-derived peptide bound to the human major histocompatibility complex class I molecule HLA-B*1501.Acta Crystallogr Sect F Struct Biol Cryst Commun. 2008 Jun 1;64(Pt 6):459-62. doi: 10.1107/S1744309108012396. Epub 2008 May 17. Acta Crystallogr Sect F Struct Biol Cryst Commun. 2008. PMID: 18540051 Free PMC article.
References
-
- Achour, A., Begue, B., Gomard, E., Paul, P., Sayagh, B., Van Pel, A. & Levy, J. P. (1986). Eur. J. Immunol.16, 597–604. - PubMed
-
- Bodmer, J., Cambon-Thomsen, A., Hors, J., Piazza, A. & Sanchez-Mazas, A. (1999). In Proceedings of the Twelfth International Histocompatibility Workshop and Conference, edited by D. Charron. Paris: EDK.
-
- Burrows, S. R., Rossjohn, J. & McCluskey, J. (2006). Trends Immunol.27, 11–16. - PubMed
-
- Buus, S., Stryhn, A., Winther, K., Kirkby, N. & Pedersen, L. O. (1995). Biochim. Biophys. Acta, 1243, 453–460. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

