Thrombospondins deployed by thrombopoietic cells determine angiogenic switch and extent of revascularization
- PMID: 17143334
- PMCID: PMC1679710
- DOI: 10.1172/JCI29314
Thrombospondins deployed by thrombopoietic cells determine angiogenic switch and extent of revascularization
Abstract
Thrombopoietic cells may differentially promote or inhibit tissue vascularization by releasing both pro- and antiangiogenic factors. However, the molecular determinants controlling the angiogenic phenotype of thrombopoietic cells remain unknown. Here, we show that expression and release of thrombospondins (TSPs) by megakaryocytes and platelets function as a major antiangiogenic switch. TSPs inhibited thrombopoiesis, diminished bone marrow microvascular reconstruction following myelosuppression, and limited the extent of revascularization in a model of hind limb ischemia. We demonstrate that thrombopoietic recovery following myelosuppression was significantly enhanced in mice deficient in both TSP1 and TSP2 (TSP-DKO mice) in comparison with WT mice. Megakaryocyte and platelet levels in TSP-DKO mice were rapidly restored, thereby accelerating revascularization of myelosuppressed bone marrow and ischemic hind limbs. In addition, thrombopoietic cells derived from TSP-DKO mice were more effective in supporting neoangiogenesis in Matrigel plugs. The proangiogenic activity of TSP-DKO thrombopoietic cells was mediated through activation of MMP-9 and enhanced release of stromal cell-derived factor 1. Thus, TSP-deficient thrombopoietic cells function as proangiogenic agents, accelerating hemangiogenesis within the marrow and revascularization of ischemic hind limbs. As such, interference with the release of cellular stores of TSPs may be clinically effective in augmenting neoangiogenesis.
Figures

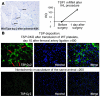
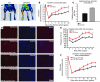
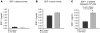
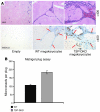
Comment in
-
The sticky truth about angiogenesis and thrombospondins.J Clin Invest. 2006 Dec;116(12):3111-3. doi: 10.1172/JCI30685. J Clin Invest. 2006. PMID: 17143327 Free PMC article.
Similar articles
-
The sticky truth about angiogenesis and thrombospondins.J Clin Invest. 2006 Dec;116(12):3111-3. doi: 10.1172/JCI30685. J Clin Invest. 2006. PMID: 17143327 Free PMC article.
-
Thrombopoietic cells and the bone marrow vascular niche.Ann N Y Acad Sci. 2007 Jun;1106:175-9. doi: 10.1196/annals.1392.004. Epub 2007 Mar 29. Ann N Y Acad Sci. 2007. PMID: 17395736 Review.
-
Thrombospondin-1 is a plasmatic marker of peripheral arterial disease that modulates endothelial progenitor cell angiogenic properties.Arterioscler Thromb Vasc Biol. 2011 Mar;31(3):551-9. doi: 10.1161/ATVBAHA.110.220624. Epub 2010 Dec 9. Arterioscler Thromb Vasc Biol. 2011. PMID: 21148423 Clinical Trial.
-
Chemokine-mediated interaction of hematopoietic progenitors with the bone marrow vascular niche is required for thrombopoiesis.Nat Med. 2004 Jan;10(1):64-71. doi: 10.1038/nm973. Epub 2003 Dec 21. Nat Med. 2004. PMID: 14702636
-
Thrombospondins as anti-angiogenic therapeutic agents.Curr Pharm Des. 2003;9(7):583-8. doi: 10.2174/1381612033391342. Curr Pharm Des. 2003. PMID: 12570805 Review.
Cited by
-
Differential changes in platelet VEGF, Tsp, CXCL12, and CXCL4 in patients with metastatic cancer.Clin Exp Metastasis. 2010 Mar;27(3):141-9. doi: 10.1007/s10585-010-9311-6. Epub 2010 Feb 25. Clin Exp Metastasis. 2010. PMID: 20182908
-
Platelets and (Lymph)angiogenesis.Cold Spring Harb Perspect Med. 2023 Jan 3;13(1):a041174. doi: 10.1101/cshperspect.a041174. Cold Spring Harb Perspect Med. 2023. PMID: 35534208 Free PMC article. Review.
-
Transplantation of Endothelial Cells to Mitigate Acute and Chronic Radiation Injury to Vital Organs.Radiat Res. 2016 Aug;186(2):196-202. doi: 10.1667/RR14461.1. Epub 2016 Jul 26. Radiat Res. 2016. PMID: 27459700 Free PMC article. Review.
-
Low-dose decitabine for refractory prolonged isolated thrombocytopenia after HCT: a randomized multicenter trial.Blood Adv. 2021 Mar 9;5(5):1250-1258. doi: 10.1182/bloodadvances.2020002790. Blood Adv. 2021. PMID: 33646303 Free PMC article. Clinical Trial.
-
Heterogeneity of the bone marrow niche.Curr Opin Hematol. 2016 Jul;23(4):331-8. doi: 10.1097/MOH.0000000000000265. Curr Opin Hematol. 2016. PMID: 27177311 Free PMC article. Review.
References
-
- Billroth, T. 1878. Lectures on surgical pathology and therapeutics. A handbook for students and practitioners. New Syndenham Society. London, United Kingdom. 355 pp.
-
- Honn K.V., Tang D.G., Crissman J.D. Platelets and cancer metastasis: a causal relationship? . Cancer Metastasis Rev. 1992;11:325–351. - PubMed
-
- Sierko E., Wojtukiewicz M.Z. Platelets and angiogenesis in malignancy. Semin. Thromb. Hemost. 2004;30:95–108. - PubMed
-
- Rhee J.S., et al. The functional role of blood platelet components in angiogenesis. Thromb. Haemost. 2004;92:394–402. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 AR45418/AR/NIAMS NIH HHS/United States
- P01 HL046403/HL/NHLBI NIH HHS/United States
- R01 NS041462/NS/NINDS NIH HHS/United States
- R01 HL59312/HL/NHLBI NIH HHS/United States
- P01 HL067839/HL/NHLBI NIH HHS/United States
- R01 HL67839/HL/NHLBI NIH HHS/United States
- R37 HL047073/HL/NHLBI NIH HHS/United States
- R01 HL61849/HL/NHLBI NIH HHS/United States
- P01 HL46403/HL/NHLBI NIH HHS/United States
- R21 HL083222/HL/NHLBI NIH HHS/United States
- R01 NS41462/NS/NINDS NIH HHS/United States
- HHMI/Howard Hughes Medical Institute/United States
- P01 HL059312/HL/NHLBI NIH HHS/United States
- R01 HL061849/HL/NHLBI NIH HHS/United States
- R01 HL075234/HL/NHLBI NIH HHS/United States
- R01 AR045418/AR/NIAMS NIH HHS/United States
- R37 HL47073/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

