Precursor binding to an 880-kDa Toc complex as an early step during active import of protein into chloroplasts
- PMID: 17144891
- PMCID: PMC1804235
- DOI: 10.1111/j.1365-313X.2006.02944.x
Precursor binding to an 880-kDa Toc complex as an early step during active import of protein into chloroplasts
Abstract
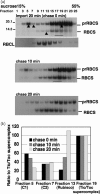
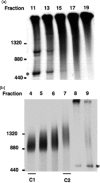
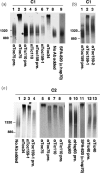
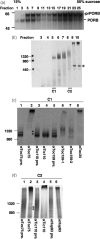
The import of protein into chloroplasts is mediated by translocon components located in the chloroplast outer (the Toc proteins) and inner (the Tic proteins) envelope membranes. To identify intermediate steps during active import, we used sucrose density gradient centrifugation and blue-native polyacrylamide gel electrophoresis (BN-PAGE) to identify complexes of translocon components associated with precursor proteins under active import conditions instead of arrested binding conditions. Importing precursor proteins in solubilized chloroplast membranes formed a two-peak distribution in the sucrose density gradient. The heavier peak was in a similar position as the previously reported Tic/Toc supercomplex and was too large to be analyzed by BN-PAGE. The BN-PAGE analyses of the lighter peak revealed that precursors accumulated in at least two complexes. The first complex migrated at a position close to the ferritin dimer (approximately 880 kDa) and contained only the Toc components. Kinetic analyses suggested that this Toc complex represented an earlier step in the import process than the Tic/Toc supercomplex. The second complex in the lighter peak migrated at the position of the ferritin trimer (approximately 1320 kDa). It contained, in addition to the Toc components, Tic110, Hsp93, and an hsp70 homolog, but not Tic40. Two different precursor proteins were shown to associate with the same complexes. Processed mature proteins first appeared in the membranes at the same fractions as the Tic/Toc supercomplex, suggesting that processing of transit peptides occurs while precursors are still associated with the supercomplex.
Figures

Similar articles
-
Stromal Hsp70 is important for protein translocation into pea and Arabidopsis chloroplasts.Plant Cell. 2010 May;22(5):1516-31. doi: 10.1105/tpc.109.071415. Epub 2010 May 18. Plant Cell. 2010. PMID: 20484004 Free PMC article.
-
Stable megadalton TOC-TIC supercomplexes as major mediators of protein import into chloroplasts.Plant J. 2017 Oct;92(2):178-188. doi: 10.1111/tpj.13643. Epub 2017 Sep 15. Plant J. 2017. PMID: 28745032
-
Characterization of the preprotein translocon at the outer envelope membrane of chloroplasts by blue native PAGE.Plant Cell Physiol. 2006 Mar;47(3):363-71. doi: 10.1093/pcp/pcj002. Epub 2006 Jan 13. Plant Cell Physiol. 2006. PMID: 16415065
-
Toc, tic, and chloroplast protein import.Biochim Biophys Acta. 2002 Jun 12;1590(1-3):177-89. doi: 10.1016/s0167-4889(02)00176-3. Biochim Biophys Acta. 2002. PMID: 12180471 Review.
-
Toc, Tic, and chloroplast protein import.Biochim Biophys Acta. 2001 Dec 12;1541(1-2):64-79. doi: 10.1016/s0167-4889(01)00147-1. Biochim Biophys Acta. 2001. Corrected and republished in: Biochim Biophys Acta. 2002 Jun 12;1590(1-3):177-89. doi: 10.1016/s0167-4889(02)00176-3. PMID: 11750663 Corrected and republished. Review.
Cited by
-
An essential role for chloroplast heat shock protein 90 (Hsp90C) in protein import into chloroplasts.Proc Natl Acad Sci U S A. 2013 Feb 19;110(8):3173-8. doi: 10.1073/pnas.1219229110. Epub 2013 Feb 4. Proc Natl Acad Sci U S A. 2013. PMID: 23382192 Free PMC article.
-
Stimulation of transit-peptide release and ATP hydrolysis by a cochaperone during protein import into chloroplasts.J Cell Biol. 2006 Dec 18;175(6):893-900. doi: 10.1083/jcb.200609172. Epub 2006 Dec 11. J Cell Biol. 2006. PMID: 17158958 Free PMC article.
-
Targeting and assembly of components of the TOC protein import complex at the chloroplast outer envelope membrane.Front Plant Sci. 2014 Jun 11;5:269. doi: 10.3389/fpls.2014.00269. eCollection 2014. Front Plant Sci. 2014. PMID: 24966864 Free PMC article. Review.
-
Stromal Hsp70 is important for protein translocation into pea and Arabidopsis chloroplasts.Plant Cell. 2010 May;22(5):1516-31. doi: 10.1105/tpc.109.071415. Epub 2010 May 18. Plant Cell. 2010. PMID: 20484004 Free PMC article.
-
New Insights into the Chloroplast Outer Membrane Proteome and Associated Targeting Pathways.Int J Mol Sci. 2022 Jan 29;23(3):1571. doi: 10.3390/ijms23031571. Int J Mol Sci. 2022. PMID: 35163495 Free PMC article. Review.
References
-
- Bauer J. The major protein import receptor of plastids is essential for chloroplast biogenesis. Nature. 2000;403:203–207. - PubMed
-
- Chacinska A, et al. Mitochondrial presequence translocase: switching between TOM tethering and motor recruitment involves Tim21 and Tim17. Cell. 2005;120:817–829. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

