Vascular abnormalities in mice deficient for the G protein-coupled receptor GPR4 that functions as a pH sensor
- PMID: 17145776
- PMCID: PMC1800706
- DOI: 10.1128/MCB.01909-06
Vascular abnormalities in mice deficient for the G protein-coupled receptor GPR4 that functions as a pH sensor
Abstract
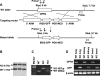
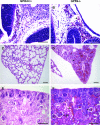
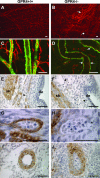
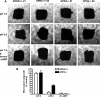
GPR4 is a G protein-coupled receptor expressed in the vasculature, lung, kidney, and other tissues. In vitro ectopic overexpression studies implicated GPR4 in sensing extracellular pH changes leading to cyclic AMP (cAMP) production. To investigate its biological roles in vivo, we generated GPR4-deficient mice by homologous recombination. Whereas GPR4-null adult mice appeared phenotypically normal, neonates showed a higher frequency of perinatal mortality. The average litter size from GPR4(-/-) intercrosses was approximately 30% smaller than that from GPR4(+/+) intercrosses on N3 and N5 C57BL/6 genetic backgrounds. A fraction of knockout embryos and neonates had spontaneous hemorrhages, dilated and tortuous subcutaneous blood vessels, and defective vascular smooth muscle cell coverage. Mesangial cells in kidney glomeruli were also significantly reduced in GPR4-null neonates. Some neonates exhibited respiratory distress with airway lining cell metaplasia. To examine whether GPR4 is functionally involved in vascular pH sensing, an ex vivo aortic ring assay was used under defined pH conditions. Compared to wild-type aortas, microvessel outgrowth from GPR4-null aortas was less inhibited by acidic extracellular pH. Treatment with an analog of cAMP, a downstream effector of GPR4, abolished microvessel outgrowth bypassing the GPR4-knockout phenotype. These results suggest that GPR4 deficiency leads to partially penetrant vascular abnormalities during development and that this receptor functions in blood vessel pH sensing.
Figures




References
-
- An, S., C. Tsai, and E. J. Goetzl. 1995. Cloning, sequencing and tissue distribution of two related G protein-coupled receptor candidates expressed prominently in human lung tissue. FEBS Lett. 375:121-124. - PubMed
-
- Berger, A. C., X. Q. Wang, A. Zalatoris, J. Cenna, and J. C. Watson. 2004. A murine model of ex vivo angiogenesis using aortic disks grown in fibrin clot. Microvasc. Res. 68:179-187. - PubMed
-
- Betsholtz, C., P. Lindblom, M. Bjarnegard, M. Enge, H. Gerhardt, and P. Lindahl. 2004. Role of platelet-derived growth factor in mesangium development and vasculopathies: lessons from platelet-derived growth factor and platelet-derived growth factor receptor mutations in mice. Curr. Opin. Nephrol Hypertens. 13:45-52. - PubMed
-
- Burbridge, M. F., D. C. West, G. Atassi, and G. C. Tucker. 1999. The effect of extracellular pH on angiogenesis in vitro. Angiogenesis 3:281-288. - PubMed
-
- Carey, A. V., R. M. Carey, and R. A. Gomez. 1992. Expression of alpha-smooth muscle actin in the developing kidney vasculature. Hypertension 19(2 Suppl.):II168-II175. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
